In 2022, clinical trials indicated that a drug called lecanemab could slow cognitive decline in people with Alzheimer’s disease; soon after the results were published, the global Alzheimer’s community heralded lecanemab as a momentous discovery. However, closer inspection of the data by independent investigators revealed that the drug might significantly help men, but not women1.
The finding is a reminder that, even though tremendous advances are being made in the clinical application of cutting-edge technologies, such as gene editing and artificial intelligence (AI), there is a remarkable lack of understanding about how many aspects of human health are affected by variables as seemingly basic as sex and gender.
Over the past decade or so, funders and publishers have made extensive efforts to encourage researchers to address the effects of sex and, in human studies, gender where appropriate. Thanks in part to these efforts, more insights are beginning to emerge. For Alzheimer’s and many other diseases that are common causes of death, including cardiovascular diseases, cancer, chronic respiratory conditions and diabetes, a person’s sex and gender can influence their risk of developing the disease, how quickly and accurately they are diagnosed, what treatment they receive and how they fare.
But even for the most-studied conditions, many questions remain. Few investigators have begun to probe the interrelationships between sex and gender, for example. And in cases in which researchers are managing to unpick the multifaceted effects of sex, this knowledge is not being sufficiently incorporated into the design of clinical trials or adequately changing the practice of medicine.
The consideration of sex and, where appropriate, gender in biological research must become routine — especially as molecular genetics, biomedical engineering and AI open up possibilities for treatments that are better tailored to the needs of individuals. Likewise, the culture of medicine must be transformed so that approaches to treatment evolve in response to the data. This will require further engagement from funders and publishers, but action from many other players, too. Pharmaceutical companies and intergovernmental organizations, among others, must acknowledge three things: how sex and gender can have huge effects on health outcomes; how these effects are often disregarded in basic research and clinical trials; and that change can come only through increasing awareness among all stakeholders of the importance of shifting the dial.
Health outcomes affected
In most human clinical records so far, sex is reported by physicians or participants in studies ticking one of two boxes: ‘female’ or ‘male’. In those clinical studies in which data are collected on chromosomes, hormone levels, reproductive anatomy or other sex characteristics, these features will frequently reflect a person’s sex assigned at birth. But this is not always the case. Added to this, sex and gender have often been used interchangeably, but they are not the same and they do not always align. Current definitions of gender include the social, psychological, cultural and behavioural aspects of being a man or woman (whether cisgender or transgender), non-binary or identifying with one or more other evolving terms2.
In several countries, new recommendations about how researchers should obtain data on people’s sex and gender should mean that, in the future, investigators will be able to more-accurately probe the roles of both in human health. But in general, there has been incomplete capture of information for sex and gender so far, including for individuals whose sex characteristics and/or gender identities don’t fall into a binary categorization scheme.

Women are more likely to die after a severe heart attack than are men.Credit: Simon Dawson/Reuters
In this article, consistent with much of the published population-wide data, we refer to a woman as someone who identifies with that gender and was assigned female sex at birth (a cis woman), and a man as someone who identifies with that gender and was assigned male sex at birth (a cis man). But we recognize that participants in the studies we describe might not have been asked about both their gender and their sex.
For all sorts of non-communicable diseases, there are differences between men and women in the average age at which they are diagnosed, the average age at which they die and even in their rates of death.
We need more-nuanced approaches to exploring sex and gender in research
Such variations, from the earlier onset of cardiovascular diseases in men to the more frequent occurrence of Alzheimer’s disease in women, might stem from differences in biology, which can affect people’s likelihood of developing a disease and how they respond to treatment. Or these discrepancies might stem from variation in people’s exposure to the environmental factors that trigger the disease, how they manage their condition, how they are treated by carers and so on, all of which can be influenced by a person’s gender. Often, a combination of factors will be at work.
Take heart attacks. Studies conducted over the past decade have revealed extensive sex differences in the expression of certain genes in heart tissue, which in turn affect the type and function of the cells that make up the heart.
Such variation could help to explain why men are likely to have a heart attack for the first time around six years earlier than women — in the United States, at 65.6 years old in men compared with 72 years old in women3 — and why (in Australia, at least) heart attacks are at least twice as common in men relative to women of comparable ages (see go.nature.com/3qbvrxq). Likewise, although mechanisms are yet to be fully understood, it is plausible that differences in people’s biology help to explain why women are more likely to experience pain between their shoulder blades, nausea or vomiting and shortness of breath during a heart attack; why men are more likely to experience chest pain and increased sweating; and why women are nearly twice as likely as are men to die after a severe heart attack.
Yet, when it comes to the risk of dying, social and environmental factors — shaped by gender — also seem to be important.
Tobacco consumption increases a person’s risk of having a heart attack, and smoking is much more common among men globally. Worldwide, around 37% of men smoke compared with around 8% of women. Also, in part because health-care professionals and others are more familiar with the heart attack symptoms commonly seen in men, when women have a heart attack, they are more likely to delay seeking help, and carers are often slower to intervene4. In fact, in a study of more than 500,000 people who experienced a heart attack and were admitted to hospital in the United Kingdom between 2004 and 2013, women were 37% more likely to receive an incorrect initial diagnosis after a severe heart attack than were men5. Even when women tell their physicians that they have chest pain, they are two to three times less likely to be referred to a cardiologist than are men6.
A similarly complicated picture has been emerging in relation to strokes7 — another cardiovascular disease — and, in the past few years, in relation to cancer.

Smoking is more common among men than women globally.Credit: Behrouz Mehri/AFP/Getty
Most cancers that occur in non-reproductive organs develop earlier in men than they do in women. In the United States, oesophageal cancer is 4.5 times more likely to occur and cause death in men than in women, for example, and lung cancers, the most common drivers of cancer-associated deaths worldwide, kill around 40% more men than women8.
Just as with heart disease and stroke, some of this variation seems to stem from behavioural differences. Tobacco consumption increases a person’s risk of developing several cancers7. For thyroid cancers, however, women are more likely to develop the disease than are men — three times more likely in some places — which suggests that other factors might drive the different rates of this particular cancer in women and men9. But tumours typically arise because of problems with cells’ genetic-repair systems, together with inadequate damage clearance, and genetic differences between men and women that affect cancers are beginning to emerge.
Male–female comparisons are powerful in biomedical research — don’t abandon them
Much more research is needed to understand how sex affects the rate at which genes mutate, cells’ capacities to repair and clear damaged DNA, and when genetic damage starts causing disease. Yet research led by one of us (S.H.) on lung adenocarcinoma, the most common type of lung cancer, suggests that women can survive for longer than men after they are diagnosed, in part thanks to cancer-defence genes in women driving more-robust immune responses10. X chromosomes encode many genes that are linked to immunity, and women with two X chromosomes might express these genes at higher levels than men with XY chromosomes.
Responses to cancer treatments also differ between men and women. Chemotherapies tend to work better in women than in men. This could be because it can take longer for women’s bodies to clear certain drugs, which could partly explain why women are also 34% more likely than men to experience harmful side effects11. Moreover, women with lung cancer typically have better outcomes after surgery, which they undergo more often than men8. This is probably due, at least in part, to women having less advanced disease when they are diagnosed than men do12. But the generally stronger immune responses in women might also help their recovery8.
Too often ignored
Despite these compelling indications that sex and gender matter, when it comes to many diseases that are leading causes of death, many researchers and health practitioners still fail to adequately take sex and gender into account. They might also be influenced by conscious or unconscious bias.
In the case of heart disease, the differences in gene expression and cellular make-up and activity found in men and women’s hearts highlight the need for sex-specific cardiac tissue models, sustained by sex-appropriate vasculature13. (Women on average have smaller hearts with narrower vessels compared with men.) Currently, researchers tend to construct heart models using either animal or human cells, but without necessarily ensuring that cells are sourced from individuals of only one sex per model. In fact, identifying sex disparities in basic heart biology is crucial to engineering relevant heart models with stem cells, for example, which investigators are now developing to aid the study of heart disease13.
For both heart disease and stroke, because of decades of under-representation of women in clinical trials, many of today’s standard treatments are based on studies of what happens in men who weigh around 70 kilograms. In clinical trials conducted for stroke and heart conditions between 2010 and 2017, women worldwide were under-enrolled relative to the prevalence of these diseases in the general population — by around 20%14. There is also significant underfunding of research for many conditions that are more prevalent in women compared with those that are more common in men (see ‘Disparities in health and disease’).
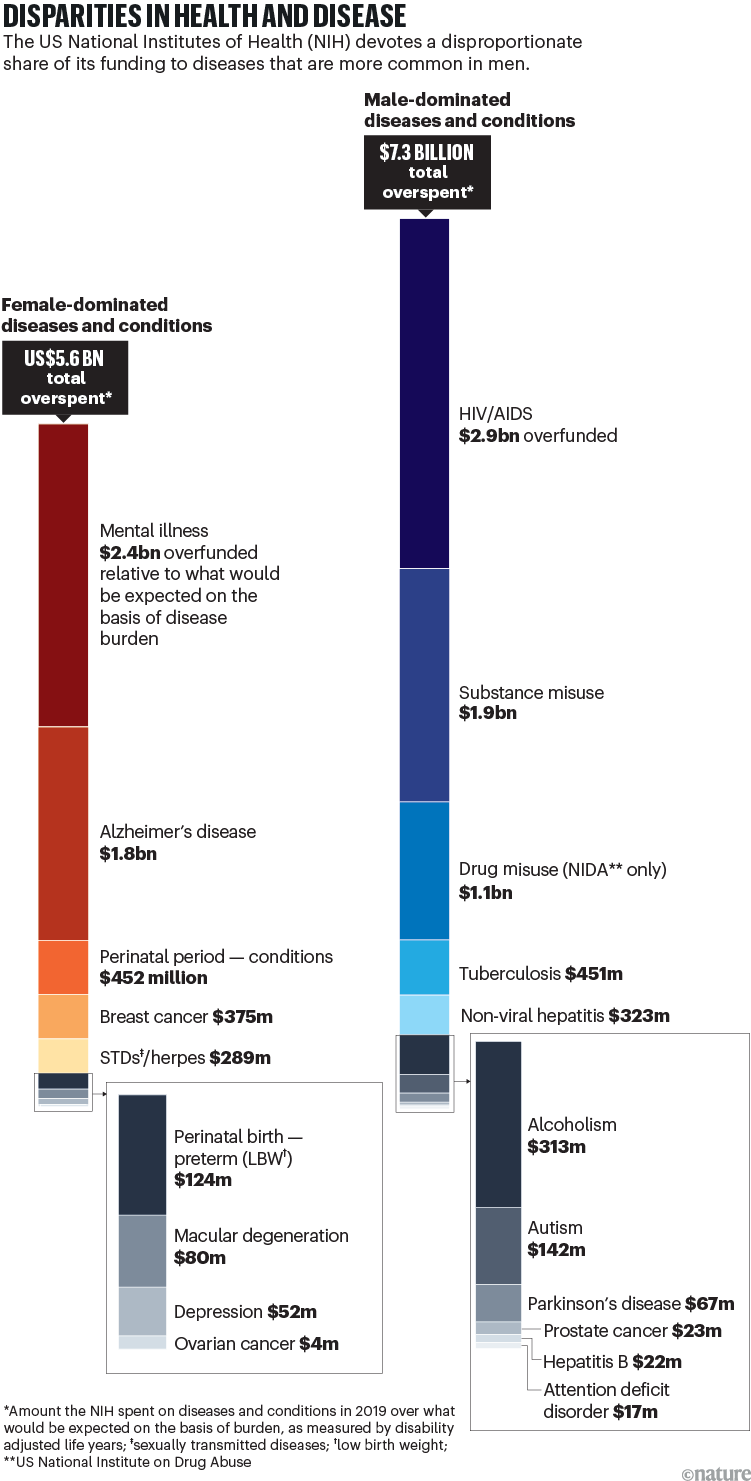
Source: A. A. Mirin J. Womens Health 30, 956–963 (2021).
Basic research on cancer is similarly riddled with problems. Take the sex of the cell lines that are stored in commercial cell banks, which have been studied for decades and are the source of much of today’s textbook knowledge. For lung cancers, male lines outnumber female lines by two to one. For liver cancers, the ratio is seven to one. Until a few years ago, few researchers studying cancer in cultured cells in the lab even considered the sex of the cells they were studying. Also, the standard media in which cells are grown is frequently supplemented with fetal calf serum from a mixture of male and female calves, and so contains both male and female sex hormones. And phenol red, a dye commonly used to monitor the pH of tissue culture media mimics the hormone oestrogen8.
To add to the difficulties, research findings that emerge from the use of these cell lines are often tested in mice of only one sex. The results of these studies are then used to guide human trials that include both men and women participants. And in oncological clinical trials, just as with stroke and heart disease, women are still under-enrolled relative to the burden of disease they experience7.
Inclusivity in human trials will ensure the best possible outcomes for all participants, including cis and trans women and men, gender-diverse and intersex people (see ‘Inclusivity in practice’). Studies are showing, for example, that circadian rhythms — which can affect heart function and might impact how drugs are metabolized — differ between men and women15. So how might they compare in non-binary or transgender people? Likewise, knowledge about the immune responses of people with atypical numbers of sex chromosomes is likely to be crucial when it comes to the use of immune checkpoint inhibitors and other immune therapies for treating cancer. Those with Klinefelter syndrome, for example, who, similar to cis women, are at a higher risk of developing breast cancer than are cis men, have multiple X chromosomes that are rich in genes involved in the immune response.
Heightened awareness
Routinely taking sex and gender into account in research and using that knowledge to change health care could benefit billions of people. So what’s needed to make this happen?
Policy changes — such as the US National Institutes of Health’s 2016 call for the inclusion of male and female sexes in studies involving cells, tissues and animals — are crucial. But for many researchers, such calls seem burdensome, especially because studying more than one sex can increase costs. (Sample sizes might need to be increased to achieve sufficient statistical power when comparing groups.)
Alongside initiatives from funders and publishers, awareness must be built — among students, researchers, clinicians, medical ethics committees, research governance bodies and community groups — of the ramifications of failing to consider sex and gender, and how to correct the problem.
Accounting for sex and gender makes for better science
Efforts led by the Canadian Institutes of Health Research (CIHR) are encouraging. Even though the permeation of knowledge from research to health care has been glacial, between 2011 and 2019, the proportion of all research grant applications submitted to the CIHR that took sex into consideration increased from around 22% to 83%. Gender as a variable is now also included in many of the human studies funded by the CIHR.
Several initiatives have contributed to this. As an example, as well as asking grant applicants to include a section in their research proposals on whether they are considering sex and gender and how they will do so, or why this is not considered applicable, the CIHR has provided training for scientists and organized workshops involving researchers and specialists in sex and gender. Applicants are more likely to receive funding if they provide a satisfactory rationale for their choices.
Convincing people in leadership roles — in governments, laboratories, medical ethics boards, education and so on — of the importance of including sex and gender in research is especially crucial. More studies demonstrating the financial costs of not doing so could help. Between 1997 and 2000, for instance, eight prescription drugs were retracted from the US market because inadequate clinical testing in women had failed to identify that the drugs put women at greater risk of developing health problems than men. This error cost pharmaceutical companies and taxpayers an estimated US$1.6 billion per drug16.
The scale of transformation needed will also require more engagement from global players.
Even as far back as 2007, the 60th World Health Assembly — the decision-making body of the World Health Organization (WHO) — passed a resolution to urge researchers to split their data according to sex and to include gender analyses where appropriate. Steps to improve care for transgender people or those with diverse genders are also starting to be taken; in December last year, the WHO established a Guideline Development Group, to provide recommendations on how to address the health of transgender and gender-diverse people. But more extensive efforts, comparable to all United Nations member states committing to target 5.b of the 2015 Sustainable Development Goals by 2030, will be crucial. (This target is to “enhance the use of enabling technology, in particular information and communications technology, to promote the empowerment of women”.)
Lastly, under the guidance of regulatory bodies such as the European Medicines Agency and the scientific entrepreneur community, the pharmaceutical industry must do more to ensure that preclinical work is robust, and that products are tested on enough people of different sexes and genders. Many leading pharmaceutical companies acknowledge on their websites the importance of including diverse groups in clinical trials, but evidence of actions to address the issue is only just emerging.
Awareness of the problems around sex and gender is growing fast. And although many are concerned that medical applications of AI will perpetuate already existing biases17, promising developments are emerging in the use of machine learning to make diagnoses that are appropriate for people’s sex and gender.
For decades, for instance, physicians worldwide have been determining whether a person has had a heart attack by using the Global Registry of Acute Coronary Events (GRACE) score, which was derived from trials mainly involving men. In 2022, the application of machine learning to data that had been split for men and women refined the predictors for women. And these revised predictors did a better job of matching individuals to appropriate interventions18.
Greater awareness, the wealth of data now emerging and the possibilities presented by new tools, from AI to gene editing, could mean a new era for research and medicine.



 Sex and gender in science
Sex and gender in science
 We need more-nuanced approaches to exploring sex and gender in research
We need more-nuanced approaches to exploring sex and gender in research
 Male–female comparisons are powerful in biomedical research — don’t abandon them
Male–female comparisons are powerful in biomedical research — don’t abandon them
 Nature journals raise the bar on sex and gender reporting in research
Nature journals raise the bar on sex and gender reporting in research
 Accounting for sex and gender makes for better science
Accounting for sex and gender makes for better science
 The fraught quest to account for sex in biology research
The fraught quest to account for sex in biology research