DNA Script co-founder Thomas Ybert with the company’s bench-top DNA printer.Credit: ERIC PIERMONT/AFP via Getty
In 2021, President Emmanuel Macron announced a €7.5-billion (US$7.9-billion) plan aimed at making France a global health-care innovation leader by 2030. It is too soon to tell whether he will achieve his ambition, but researchers on the ground say that the sector is growing and maturing. Between 2020 and 2022, the number of health-technology companies grew from 2,050 to 2,640, according to a survey by France Biotech, an industry body based in Paris.
Furthermore, the proportion of enterprises with at least one subsidiary abroad rose from one in five in 2021 to one in four last year, and the percentage of medical-technology and diagnostics companies with products on the market grew by 6 percentage points, to 56%.
More than one-quarter of the companies in France Biotech’s survey were based in the greater Paris region. Every year, Nature publishes rankings of institutions and countries according to the number of scientific articles and papers published in high-quality journals. In the 2021 Nature Index, researchers at institutions in the Paris metropolitan area accounted for 44% of France’s total research output and for 48% of its output in the biological sciences. Here, leading figures at three companies discuss the benefits and downsides of launching and running medical-technology businesses in the French capital.
Resolve Stroke
People recovering in hospital from strokes and traumatic brain injuries must be monitored in case they develop complications or brain damage. But all of the current monitoring methods have disadvantages.
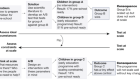
For those with the most severe injuries, probes can be inserted through the skull to track measures such as brain temperature, cerebral blood flow and intracranial pressure, but this technique requires a high level of expertise, is expensive and carries the risk of bleeds and infections. Non-invasive technologies such as transcranial doppler ultrasound, together with the tracking of vital signs such as blood pressure and heart rate, are cheaper and less risky than invasive techniques but provide less detail about the situation. Computed tomography (CT) scanners can generate higher-resolution images, but are large and expensive, and patients must be transported to them.
The Paris-based start-up firm Resolve Stroke is developing a bedside neuromonitoring device that it hopes will “combine the benefits of transcranial doppler and CT scanners”, says co-founder and chief technology officer Vincent Hingot. “Our device will enable measurements to be taken by non-experts and provide more detailed information than conventional doppler,” he says. “Given sufficient time, it could be used as an alternative to CT scans in some cases, reducing X-ray exposures and intra-hospital transport, and enabling more frequent imaging.”
Conventional medical ultrasound generates images of organs by emitting sound waves and recording their echoes when they hit structures in the body. It is widely used, but cannot produce high-resolution images of the brain and other organs that are deep inside the body.
But in 2015, a team led by physicists Olivier Couture and Mickael Tanter at the Langevin Institute in ESPCI-ParisTech showed it was possible to increase the resolution tenfold. The researchers injected bubbles of inert gas with diameters of 1–5 micrometres into the bloodstream and tracked them as they moved around the body, capturing 1,000 images a second1. Last May, Couture, now at Sorbonne University in Paris, Hingot and biomedical engineer Aritz Zamacola launched Resolve Stroke to commercialize technology based on the technique, called ultrasound localization microscopy. Together with physical tests, CT and magnetic resonance imaging (MRI) scans are widely used to determine whether strokes have been caused by blocked arteries or by burst blood vessels, and therefore what type of treatment is required. The flow of blood and oxygen to the brain must be restored within 3–4.5 hours to minimize the risks of permanent disability or death.
Hingot and his colleagues are investigating the possibility of using a portable version of their technology in ambulances to speed up stroke diagnosis and reduce the risk of patients failing to get the treatment they need in time. They think the technology could also improve the imaging and treatment of conditions such as arteriosclerosis and kidney and liver diseases.
Resolve Stroke received substantial support from both Sorbonne University and Bpifrance, a French public investment bank, to launch at the publicly funded Agoranov science and technology incubator in Paris.
Hingot says that start-ups launching in the French capital enjoy some key benefits. “It is easy to start a company in Paris,” he says. “The concentration of top universities and research groups means it attracts a lot of talent. There are also more in-person events and networking opportunities here.”
Paris also has disadvantages as a place to start a medical-technology business, according to Hingot. “While French government agencies and universities provide a lot of support in the form of funding, subsidies and access to facilities, competition for these is greater in the capital because it is home to a large number of start-ups and the higher costs of office space.”
Outlook: Research commercialization
Some of the challenges faced by Resolve Stroke relate more to being in France and Europe than specifically in Paris. The company will require approval for its clinical-trial design from the French National Agency for the Safety of Medicines and Health products. To launch a product in the European Union, it will need the go-ahead from a ‘notified body’ to check the product conforms to the relevant legal and technical requirements. Hingot says that delays in either process could put his company at a disadvantage against competitors elsewhere in the world.
Last month, Resolve Stroke announced it had secured €2.2 million in seed funding. The financing round was led by two Paris-based venture-capital companies, OVNI Capital and Quantonation, but Hingot says that the company might have to consider moving some of its operations away from Paris as it grows and gets closer to launching its products. “We are still young and we have found investors willing to take a risk on us,” he says. “However, at some point, we may have to move at least some of our activities to the United States or somewhere where greater volumes of investment funds are available. The US market is a prime target for us.”
DNA Script
Short strands of synthetic DNA, or oligos, have a growing range of applications, from life-sciences research and genetic testing to gene therapy and the production of mRNA vaccines. Since the 1980s, they have been made by sequentially linking nucleotides using a chemical technique called phosphoramidite synthesis.
The technique works well for producing short sequences of genetic material, but not for ambitious projects, such as building synthetic genes2.
“We are reaching a plateau with phosphoramidite synthesis,” says Thomas Ybert, chief executive and co-founder of Paris-based DNA Script. “It is not possible to ensure that all of the molecules undergo the reactions needed to make the nucleotides in the DNA chain, and the longer the oligo, the more errors build up.”
Changing old viticulture for all the right rieslings
DNA Script is one of several companies turning to terminal deoxynucleotidyl transferase (TdT), a naturally occurring enzyme that is particularly useful for DNA synthesis because it does not need a DNA template to copy sequences from, but that usually adds nucleotides to the end of the growing DNA sequence indiscriminately. The company engineered TdT such that it could control the order in which nucleotides are added.
In 2021, DNA Script launched SYNTAX, one of the world’s first DNA printers, which uses TdT technology to make up to 96 oligos of up to 120 nucleotides long within 24 hours. It saves users time, because they no longer have to order the oligos from commercial suppliers and wait for them to be made and shipped. “Scientists with SYNTAX in their labs have full control and the fastest available access to DNA for their projects, allowing them to iterate and make decisions faster, and generate huge gains in productivity and efficiency,” says Ybert.
DNA Script has around 220 employees, launched in Paris in 2014, and opened an office in San Francisco, California, in 2019. Ybert agrees with Hingot that Paris is a good place for companies to begin their journey, but becomes less convenient as they grow in size.
“Paris is at the centre of French culture, and so is a great place for companies seeking to attract foreign talent,” says Ybert. “Public transport is cheap, dense and frequent, which is attractive to young workers.” Furthermore, he adds, companies are closer to government officials, so have higher visibility than elsewhere in France. However, they can find it difficult to attract the sums needed to take them to the next level.
The precautionary principle, which urges caution in the face of potential risks in the absence of evidence, was incorporated into the French national constitution to apply to environmental policies in 2005. Ybert says that this has become a broader national mindset that can sometimes hold back innovation. “The French mentality is that if something is too big or new or futuristic, people can be scared and put off from doing it.” And this includes French investors, whose business is to take risks. They are not willing to take the same level of risk as do those in Silicon Valley, he explains.
Ybert says that the French government could boost innovation by lowering business taxes and introducing reforms to reduce labour costs. “The standard 35-hour working week is not competitive compared to other key economies like the US, China and other countries in the EU,” he says. “The government could also boost innovation by reducing taxes on employee shareholders so they can get higher financial returns on the risks and hard work they put in, especially for innovative ‘deep tech’ enterprises using advanced science and engineering to solve complex problems.”
Owkin
Machine learning has the potential to diagnose a number of medical conditions as well as or more accurately than humans can. Its ability to do so is, however, restricted by the data it is trained on. And for rare conditions, especially, it can be hard to access enough data.
Computer scientists at medical artificial-intelligence company Owkin are among those who say the answer lies in federated learning — in which AI models are trained on data from multiple sites without compromising privacy and security. In a paper published in January, researchers showed they had trained a machine-learning model on real patient data from two French hospitals without needing to send those data to an external location3.
France’s research minister has a plan to shake up science
The researchers used Owkin’s Substra software and high-resolution digital images of tumour biopsies to train a deep-learning model to predict the effectiveness of a chemotherapy drug in treating people with a condition known as triple-negative breast cancer, which has more-limited treatment options than other forms of breast cancer. When the model was then tested on data from the same two hospitals and two others, with a combined total of 237 patients, it outperformed a machine-learning model trained on the clinical data typically used by pathologists, including the physical characteristics of tumours and immune-system markers.
If clinicians could predict who will respond, they could adapt their treatment, instead of just treating everyone the same and hoping it will work, as they do currently, says Jean-Philippe Vert, head of research and development at Owkin.
Vert agrees that the medical-technology sector is thriving in Paris in part because the French capital provides a fertile ecosystem for those seeking employees and collaborators to help them launch companies. France came sixth globally in the 2023 Nature Index ranking of countries according to the health-sciences research output in high-quality medical journals. And the Assistance Publique–Hôpitaux de Paris, a university hospital trust based in the French capital, was one of only two non-US health-care institutions to make the latest Nature Index global top 15 for research output. “I think the medtech sector in Paris is growing quickly,” says Vert. “The academic setting is among the best in the world, with a lot of good students, big hospitals and excellent biomedical research centres. We also have big pharmaceutical companies, like Sanofi, and many mid-sized pharmas too.”
Owkin, which employs around 350 people, has raised more than $300 million in funding from Sanofi and other biopharmaceutical companies and from venture-capital funds including Google Ventures and Fidelity Investments. Vert says that Owkin’s New York offices have helped it to attract investment, highlighting how the relative lack of domestic venture capital is often a problem for French companies. “Attracting investment funding, especially at the growth stage, is easier in the US than in Paris.”


 Science in France
Science in France
 Outlook: Research commercialization
Outlook: Research commercialization
 Changing old viticulture for all the right rieslings
Changing old viticulture for all the right rieslings
 France’s research minister has a plan to shake up science
France’s research minister has a plan to shake up science
 Starting up in science: two biologists struggle to launch their labs
Starting up in science: two biologists struggle to launch their labs