- NEWS AND VIEWS
30 years of the iron hypothesis of ice ages
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 578, 370-371 (2020)
doi: https://doi.org/10.1038/d41586-020-00393-x
References
Barnola, J. M., Raynaud, D., Korotkevich, Y. S. & Lorius, C. Nature 329, 408–414 (1987).
Martin, J. H. Paleoceanography 5, 1–13 (1990).
Sarmiento, J. L., Toggweiler, J. R. & Najjar, R. Phil. Trans. R. Soc. Lond. A 325, 3–21 (1988).
De Angelis, M., Barkov, N. I. & Petrov, V. N. Nature 325, 318–321 (1987).
Cullen, J. J. Limnol. Oceanogr. 36, 1578–1599 (1991).
Hart, T. J. Discov. Rep. VIII, 1–268 (1934).
Gordon, R. M., Martin, J. H. & Knauer, G. A. Nature 299, 611–612 (1982).
Boyd, P. W. et al. Science 315, 612–617 (2007).
Anderson, R. F. & Henderson, G. M. Oceanography 18, 76–79 (2005).
Tagliabue, A. et al. Nature 543, 51–59 (2017).
Watson, A. J., Bakker, D. C. E., Ridgwell, A. J., Boyd, P. W. & Law, C. S. Nature 407, 730–733 (2000).
Kohfeld, K. E., Le Quéré, C., Harrison, S. P. & Anderson, R. F. Science 308, 74–78 (2005).
Boyle, E. A. Nature 331, 55–56 (1988).
Jaccard, S. L., Galbraith, E. D., Martínez-García, A. & Anderson, R. F. Nature 530, 207–210 (2016).
Yamamoto, A., Abe-Ouchi, A., Ohgaito, R., Ito, A. & Oka, A. Clim. Past 15, 981–996 (2019).
Bereiter, B. et al. Geophys. Res. Lett. 42, 542–549 (2015).
Wolff, E. W. et al. Nature 440, 491–496 (2006).

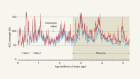
 Fingerprints of a trace nutrient
Fingerprints of a trace nutrient
 The great iron dump
The great iron dump