- NEWS AND VIEWS
Molecular architecture of the key precursor of thyroid hormones revealed
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 578, 520-521 (2020)
doi: https://doi.org/10.1038/d41586-020-00244-9
References
Citterio, C. E., Targovnik, H. M. & Arvan, P. Nature Rev. Endocrinol. 15, 323–338 (2019).
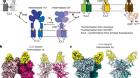
Coscia, F. et al. Nature 578, 627–630 (2020).
Portulano, C., Paroder-Belenitsky, M. & Carrasco, N. Endocr. Rev. 35, 106–149 (2014).
Dai, G., Levy, O. & Carrasco, N. Nature 379, 458–460 (1996).
Veneziani, B. M., Giallauria, F. & Gentile, F. Biochimie 81, 517–525 (1999).
Holzer, G. et al. J. Biol. Chem. 291, 16553–16566 (2016).
Dunn, A. D., Corsi, C. M., Myers, H. E. & Dunn J. T. J. Biol. Chem. 273, 25223–25229 (1998).
Dunn, J. T. & Dunn, A. D. Biochimie 81, 505–509 (1999).
Taurog, A., Dorris, M. L. & Lamas, L. Endocrinology 94, 1286–1294 (1974).
Citterio, C. E., Morishita, Y., Dakka, N., Veluswamy, B. & Arvan, P. J. Biol. Chem. 293, 4860–4869 (2018).

 Read the paper: The structure of human thyroglobulin
Read the paper: The structure of human thyroglobulin
 Signal locked in
Signal locked in
 Amyloid-β ‘seeds’ in old vials of growth hormone
Amyloid-β ‘seeds’ in old vials of growth hormone