- OUTLOOK
Building a better malaria vaccine
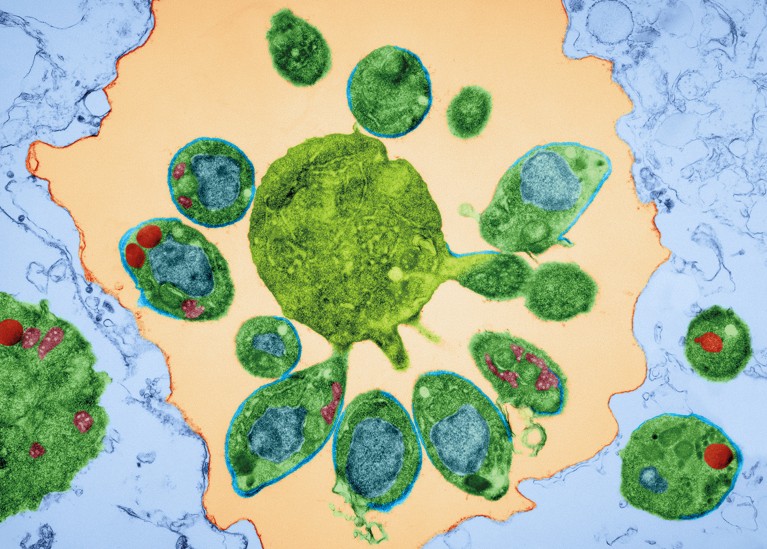
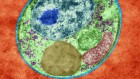
Transmission electron micrograph of merozoite-stage malarial parasites, which have caused a red blood cell to rupture. Credit: DENNIS KUNKEL MICROSCOPY/SPL
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 575, S51-S54 (2019)
doi: https://doi.org/10.1038/d41586-019-03639-5
This article is part of Nature Outlook: Vaccines, an editorially independent supplement produced with the financial support of third parties. About this content.
References
RTS,S Clinical Trials Partnership Lancet 386, 31–45 (2015).
Venkatraman, N. et al. Preprint at medRxiv https://doi.org/10.1101/19009282 (2019).
Ogwang, C. et al. Sci. Transl. Med. 7, 286re5 (2015).
Seder, R. A. Science 341, 1359–1365 (2013).
Mordmüller, B. Nature 542, 445–449 (2017).
Cohen, S, McGregor, I. A. & Carrington, S. Nature 192, 733–737 (1961).
Stanisic, D. I. et al. BMC Med. 16, 184 (2018).
Sagara, I. et al. Lancet Infect. Dis. 18, 969–982 (2018).

 Arming the immune system
Arming the immune system
 Research round-up: Vaccines
Research round-up: Vaccines
 Tailoring vaccines for older people and the very young
Tailoring vaccines for older people and the very young
 Only vaccines can eradicate parasitic worms
Only vaccines can eradicate parasitic worms
 How plants and insects inherit immunity from their parents
How plants and insects inherit immunity from their parents
 Don’t demonize parents who are hesitant to vaccinate — discuss their worries instead
Don’t demonize parents who are hesitant to vaccinate — discuss their worries instead
 The case for mandatory vaccination
The case for mandatory vaccination