- NEWS AND VIEWS
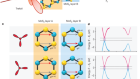
Quantum dots realize their potential
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 575, 604-605 (2019)
doi: https://doi.org/10.1038/d41586-019-03607-z
References
Ekimov, A. I. & Onushchenko, A. A. JETP Lett. 34, 345–349 (1981).
Rossetti, R., Nakahara, S. & Brus, L. E. J. Chem. Phys. 79, 1086–1088 (1983).
Won, Y.-H. et al. Nature 575, 634–638 (2019).
Efros, A. L. & Efros, Al. L. Sov. Phys. Semicond. 16, 772–775 (1982).
Brus, L. E. J. Chem. Phys. 79, 5566–5571 (1983).
Murray, C. B., Norris, D. J. & Bawendi, M. G. J. Am. Chem. Soc. 115, 8706–8715 (1993).
Hines, M. A. & Guyot-Sionnest, P. J. Phys. Chem. 100, 468–471 (1996).
Efros, Al. L. et al. Phys. Rev. B 54, 4843–4856 (1996).
Efros, Al. L. & Nesbitt, D. J. Nature Nanotechnol. 11, 661–671 (2016).
Cragg, G. E. & Efros, Al. L. Nano Lett. 10, 313–317 (2010).
Lim, J., Park, Y.-S. & Klimov, V. I. Nature Mater. 17, 42–49 (2018).

 Read the paper: Highly efficient and stable InP/ZnSe/ZnS quantum dot light-emitting diodes
Read the paper: Highly efficient and stable InP/ZnSe/ZnS quantum dot light-emitting diodes
 Wearable graphene sensors use ambient light to monitor health
Wearable graphene sensors use ambient light to monitor health
 Efficiency breakthrough for radical LEDs
Efficiency breakthrough for radical LEDs