- NEWS AND VIEWS
A breakthrough method that became vital to neuroscience
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 575, 38-39 (2019)
doi: https://doi.org/10.1038/d41586-019-02836-6
References
Neher, E. & Sakmann, B. Nature 260, 799–802 (1976).
Katz, B. & Miledi, R. J. Physiol. (Lond.) 224, 665–699 (1972).
Anderson, C. R. & Stevens, C. F. J. Physiol. (Lond.) 235, 655–691 (1973).
Hamill, O. P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F. J. Pflügers Arch. Ges. Physiol. 391, 85–100 (1981).
Hodgkin, A. L. & Huxley, A. F. J. Physiol. (Lond.) 117, 500–544 (1952).
Ahern, C. A., Payandeh, J., Bosmans, F. & Chanda, B. J. Gen. Physiol. 147, 1–24 (2016).
Wang, Y., Gupta, A., Toledo-Rodriguez, M., Wu, C. Z. & Markram, H. Cereb. Cortex 12, 395–410 (2002).
Neher, E. & Marty, A. Proc. Natl Acad. Sci. USA 79, 6712–6716 (1982).
Stuart, G. J. & Sakmann, B. Nature 367, 69–72 (1994).
Borst, J. G., Helmchen, F. & Sakmann, B. J. Physiol. (Lond.) 489, 825–840 (1995).
Reyes, A. et al. Nature Neurosci. 1, 279–285 (1998).
Smith, S. L., Smith, I. T., Branco, T. & Häusser, M. Nature 503, 115–120 (2013).
Jouhanneau, J.-S. & Poulet, J. F. A. Front. Synaptic Neurosci. 11, 15 (2019).
Lee, A. K., Manns, I. D., Sakmann, B. & Brecht, M. Neuron 51, 399–407 (2006).
Barral, J. & Reyes, A. D. Nature Neurosci. 19, 1690–1696 (2016).
Catterall, W. A. Annu. Rev. Pharmacol. Toxicol. 54, 317–338 (2014).
Daghsni, M. et al. Brain Behav. 8, e00978 (2018).
Kim, C. K., Adhikari, A. & Deisseroth, K. Nature Rev. Neurosci. 18, 222–235 (2017).
Berry, M. H. et al. Nature Commun. 10, 1221 (2019).

 10 extraordinary Nature papers
10 extraordinary Nature papers
 The paper: Single-channel currents recorded from membrane of denervated frog muscle fibres
The paper: Single-channel currents recorded from membrane of denervated frog muscle fibres
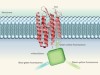 Fluorescent boost for voltage sensors
Fluorescent boost for voltage sensors
 Neurons recorded en masse
Neurons recorded en masse