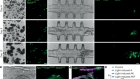
- NEWS AND VIEWS
Absent forebrain replaced by embryonic stem cells
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 563, 44-45 (2018)
doi: https://doi.org/10.1038/d41586-018-06933-w
References
Capecchi, M. R. Nature Med. 7, 1086–1090 (2001).
Chang, A. N. et al. Nature 563, 126–130 (2018).
Chen, J., Lansford, R., Stewart, V., Young, F. & Alt, F. W. Proc. Natl Acad. Sci. USA 90, 4528–4532 (1993).
Liégeois, N. J., Horner, J. W. & DePinho, R. A. Proc. Natl Acad. Sci. USA 93, 1303–1307 (1996).
Usui, J. et al. Am. J. Pathol. 180, 2417–2426 (2012).
Fraidenraich, D. et al. Science 306, 247–252 (2004).
Kobayashi, T. et al. Cell 142, 787–799 (2010).
Corbo, J. C. et al. J. Neurosci. 22, 7548–7557 (2002).
Paşca, S. P. Nature 553, 437–445 (2018).
Farahany, N. A. et al. Nature 556, 429-432 (2018).
Hyun, I. et al. Cell Stem Cell 1, 159–163 (2007).
Cohen, M. A. et al. Proc. Natl Acad. Sci. USA 113, 1570–1575 (2016).
Wu, J. et al. Cell 168, 473–486 (2017).

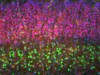 Read the paper: Neural blastocyst complementation enables mouse forebrain organogenesis
Read the paper: Neural blastocyst complementation enables mouse forebrain organogenesis
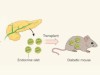 Interspecies pancreas transplanted
Interspecies pancreas transplanted
 Thirty-five years of endless cell potential
Thirty-five years of endless cell potential