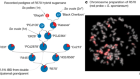
- NEWS AND VIEWS
Improved nutrient use gives cereal crops a boost
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 560, 563-564 (2018)
doi: https://doi.org/10.1038/d41586-018-05928-x
References
Li, S. et al. Nature 560, 595–600 (2018).
Peng, J. et al. Nature 400, 256–261 (1999).
Sasaki, A. et al. Nature 416, 701–702 (2002).
Gooding, M. J., Addisu, M., Uppal, R. K., Snape, J. W. & Jones, H. E. J. Agric. Sci. 150, 3–22 (2012).
Che, R. et al. Nature Plants 2, 15195 (2015).
Duan, P. et al. Nature Plants 2, 15203 (2015).
Hu, J. et al. Mol. Plant 8, 1455–1465 (2015).
Sun, P. et al. J. Integr. Plant Biol. 58, 836–847 (2016).

 Read the paper: Modulating plant growth–metabolism coordination for sustainable agriculture
Read the paper: Modulating plant growth–metabolism coordination for sustainable agriculture
 Fifty years of C4 photosynthesis
Fifty years of C4 photosynthesis
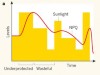 Crops on the fast track for light
Crops on the fast track for light