- NEWS AND VIEWS
When sex differences lead to extinction
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 556, 315-316 (2018)
doi: https://doi.org/10.1038/d41586-018-04059-7
References
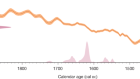
Martins, M. J. F., Puckett, T. M., Lockwood, R., Swaddle, J. P. & Hunt, G. Nature 556, 366–369 (2018).
Whitlock, M. C. Evolution 54, 1855–1861 (2000).
Mank, J. E. Nature Rev. Genet. 18, 721–730 (2017).
Le Galliard, J.-F., Fitze, P. S., Ferrière, R. & Clobert, J. Proc. Natl Acad. Sci. USA 102, 18231–18236 (2005).
Kokko, H. & Brooks, R. Ann. Zool. Fennici 40, 207–219 (2003).
Warren, W. C. et al. Nature Ecol. Evol. 2, 669–679 (2018).
Lumley, A. J. et al. Nature 522, 470–473 (2015).
Morrow, E. H. & Fricke, C. Proc. R. Soc. Lond. B 271, 2395–2401 (2004).
Doherty, P. F. et al. Proc. Natl Acad. Sci. USA 100, 5858-5862 (2003).
Park, A. W., Vandekerhove, J. & Michalakis, Y. J. Evol. Biol. 27, 1650–1661 (2014).

 Read the paper: High male sexual investment as a driver of extinction in fossil ostracods
Read the paper: High male sexual investment as a driver of extinction in fossil ostracods
 Beauty varies with the light
Beauty varies with the light
 A tip of the hat to evolutionary change
A tip of the hat to evolutionary change