- NEWS AND VIEWS
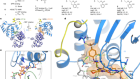
Machine learning classifies cancer
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 555, 446-447 (2018)
doi: https://doi.org/10.1038/d41586-018-02881-7
References
Capper, D. et al. Nature 555, 469–474 (2018).
Bailey, P. & Cushing, H. A Classification of the Tumors of the Glioma Group on a Histo-Genetic Basis with a Correlated Study of Prognosis (Lippincott, 1926).
Cancer Genome Atlas Research Network. N. Engl. J. Med. 372, 2481–2498 (2015).
Eckel-Passow, J. E. et al. N. Engl. J. Med. 372, 2499–2508 (2015).
Sturm, D. et al. Cell 164, 1060–1072 (2016).
Yan, H. et al. N. Engl J. Med. 360, 765–773 (2009).
Sturm, D. et al. Cancer Cell 22, 425–437 (2012).
Louis, D. N., Ohgaki, H., Wiestler, O. D. & Cavenee, W. K. (eds) WHO Classification of Tumours of the Central Nervous System 4th edn (International Agency For Research on Cancer, 2016).
Aldape, K., Nejad, R., Louis, D. N. & Zadeh, G. Neuro Oncol. 19, 336–344 (2017).
Kleppe, A. et al. Lancet Oncol. 19, 356–369 (2018).
Ehteshami Bejnordi, B. et al. J. Am. Med. Assoc. 318, 2199–2210 (2017).
Turcan, S. et al. Nature 483, 479–483 (2012).
Schwartzentruber, J. et al. Nature 482, 226–231 (2012).
Wiestler, B. et al. Acta Neuropathol. 128, 561–571 (2014).
Sahm, F. et al. Lancet Oncol. 18, 682–694 (2017).
Korshunov, A. et al. Acta Neuropathol. 134, 965–967 (2017).

 Read the paper: DNA methylation-based classification of central nervous system tumours
Read the paper: DNA methylation-based classification of central nervous system tumours
 The final frontier in cancer diagnosis
The final frontier in cancer diagnosis
 Spot the difference
Spot the difference