- NEWS AND VIEWS
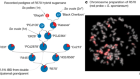
A cellular passage to the root interior
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 555, 454-455 (2018)
doi: https://doi.org/10.1038/d41586-018-02861-x
References
Kroemer, H. Wurzelhaut: Hypodermis und Endodermis der Angiospermenwurzel (Nägele, 1903).
Peterson, C. A. & Enstone, D. E. Physiol. Plant. 97, 592–598 (1996).
Wu, H., Jaeger, M., Wang, M., Li, B. & Zhang, B. G. Ann. Bot. 107, 843–853 (2011).
Robbins, N. E. II, Trontin, C., Duan, L. & Dinneny, J. R. Plant Physiol. 166, 551–559 (2014).
Andersen, T. G. et al. Nature 555, 529–533 (2018).
Barberon, M. et al. Cell 164, 447–459 (2016).
Mähönen A. P. et al. Science 311, 94–98 (2006).
De Rybel, B. et al. Science 345, 1255215 (2014).
Bishopp, A. et al. Curr. Biol. 21, 917–926 (2011).
el-Showk, S. et al. PLoS Comput. Biol. 10, e1004450 (2015).
Hamburger, D., Rezzonico, E., MacDonald-Comber Petétot, J., Somerville, C. & Poirier, Y. Plant Cell 14, 889–902 (2002).

 Read the paper: Diffusible repression of cytokinin signalling produces endodermal symmetry and passage cells
Read the paper: Diffusible repression of cytokinin signalling produces endodermal symmetry and passage cells
 Curating communities from plants
Curating communities from plants
 An immunity boost combats crop disease
An immunity boost combats crop disease