- NEWS AND VIEWS
Burst firing sets the stage for depression
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 554, 304-305 (2018)
doi: https://doi.org/10.1038/d41586-018-01588-z
References
Berridge, K. C. & Robinson, T. E. Brain Res. Brain Res. Rev. 28, 309–369 (1998).
Di Chiara, G. & Bassareo, V. Curr. Opin. Pharmacol. 7, 69–76 (2007).
Schultz, W. Neuron 36, 241–263 (2002).
Christoph, G. R., Leonzio, R. J. & Wilcox, K. S. J. Neurosci. 6, 613–619 (1986).
Ji, H. & Shepard, P. D. J. Neurosci. 27, 6923–6930 (2007).
Matsumoto, M. & Hikosaka, O. Nature 447, 1111–1115 (2007).
Sartorius, A. et al. Biol. Psychiatry 67, e9–e11 (2010).
Yang, Y. et al. Nature 554, 317–322 (2018).
Cui, Y. et al. Nature 554, 323–327 (2018).
Grace, A. A., Floresco, S. B., Goto, Y. & Lodge, D. J. Trends Neurosci. 30, 220–227 (2007).
Berman, R. M. et al. Biol. Psychiatry 47, 351–354 (2000).
Halassa, M. M. & Haydon, P. G. Annu. Rev. Physiol. 72, 335-355 (2010).
Lammel, S., Ion, D. I., Roeper, J. & Melenka, R. C. Neuron 70, 855–862 (2011).
Molofsky, A. V. et al. Genes Dev. 26, 891–907 (2012).
Ransom, B., Behar, T. & Nedergaard, M. Trends Neurosci. 26, 520–522 (2003).
Autry, A. E. et al. Nature 475, 91–95 (2011).
Zanos, P. et al. Nature 533, 481–486 (2016).
Li, N. et al. Science 329, 959–964 (2010).

 Read the paper: Ketamine blocks bursting in the lateral habenula to rapidly relieve depression
Read the paper: Ketamine blocks bursting in the lateral habenula to rapidly relieve depression
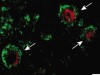 Read the paper: Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression
Read the paper: Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression
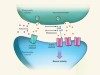 Ketamine steps out of the darkness
Ketamine steps out of the darkness
 The best way forward
The best way forward