Abstract
Emerging evidence suggests that E3 ligases play critical roles in diverse biological processes, including innate immune responses in plants. However, the mechanism of the E3 ligase involvement in plant innate immunity is unclear. We report that a rice gene, OsBBI1, encoding a RING finger protein with E3 ligase activity, mediates broad-spectrum disease resistance. The expression of OsBBI1 was induced by rice blast fungus Magnaporthe oryzae, as well as chemical inducers, benzothiadiazole and salicylic acid. Biochemical analysis revealed that OsBBI1 protein possesses E3 ubiquitin ligase activity in vitro. Genetic analysis revealed that the loss of OsBBI1 function in a Tos17-insertion line increased susceptibility, while the overexpression of OsBBI1 in transgenic plants conferred enhanced resistance to multiple races of M. oryzae. This indicates that OsBBI1 modulates broad-spectrum resistance against the blast fungus. The OsBBI1-overexpressing plants showed higher levels of H2O2 accumulation in cells and higher levels of phenolic compounds and cross-linking of proteins in cell walls at infection sites by M. oryzae compared with wild-type (WT) plants. The cell walls were thicker in the OsBBI1-overexpressing plants and thinner in the mutant plants than in the WT plants. Our results suggest that OsBBI1 modulates broad-spectrum resistance to blast fungus by modifying cell wall defence responses. The functional characterization of OsBBI1 provides insight into the E3 ligase-mediated innate immunity, and a practical tool for constructing broad-spectrum resistance against the most destructive disease in rice.
Similar content being viewed by others
Introduction
Plants have developed a precisely regulated innate immune machinery to defend themselves against potential microbial attack. Recent studies have revealed that innate immune system in plants comprises two basic inducible defence responses 1, 2, 3, 4, 5. The first layer of innate immune response is activated on detection of conserved pathogen- or microbe-associated molecular patterns (PAMPs/MAMPs) by cell surface pattern-recognition receptors (PRRs), resulting in PAMP/MAMP-triggered immunity (PTI). The second layer of innate immune response is activated on recognition of pathogen-secreted effectors by host disease resistance (R) gene-encoding proteins, leading to race-specific effector-triggered immunity (ETI). Similar signalling events are involved in regulating PTI and ETI, including changes in cellular redox status and cytoplasmic Ca2+ levels, modification of specific proteins (e.g., phosphorylation), generation of signalling molecules (e.g., salicylic acid (SA), jasmonic acid (JA), ethylene (ET), nitric oxide, and reactive oxygen species (ROS)), induction of defence-related genes, and reinforcement of cell walls 1, 2, 3, 4, 5, 6. Despite their importance in defence responses, our knowledge on the molecular mechanisms of PTI and ETI is still limited.
Blast disease, caused by Magnaporthe oryzae, is the most serious constraint on rice production. Significant progress has been made in the last decade on understanding the molecular basis of innate immune response in rice against M. oryzae by applying combined molecular and genomic approaches. Nine blast R genes were cloned and characterized 7. The recognition of M. oryzae-secreted effector protein Avr-Pita by the R protein Pita inside the rice cell could activate innate immune response 8. Two R genes, Pi9 and Pi21, confer broad-spectrum resistance against multiple races of M. oryzae 9, 10. Rac GTPase-mediated events (e.g., generation of ROS), activation of MAP kinase, and other key components, such as RAR1, SGT1, Hsp90, and WRKY45, play important roles in regulating the immune response against M. oryzae 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24. Endogenous SA is involved in defence against M. oryzae 25. A germin-like protein gene family acts as a complex quantitative trait locus conferring broad-spectrum resistance against multiple races of M. oryzae 26. However, extensive studies are required to elucidate the precise molecular mechanism of immune response in rice against M. oryzae, which could provide tools for transgenic improvement of rice blast resistance.
In studies aimed at elucidating the molecular basis of plant immune response, one main strategy is approaches based on identifying novel proteins resulted from the induction of gene expression, in addition to map-based cloning of R and mutant genes. Recent studies showed that protein degradation is one of the most important biochemical events that play critical roles in regulating immune response. The ubiquitin (Ubi)/26S proteasome system constitutes a primary pathway for degrading proteins in eukaryotes. It starts with the ubiquitination of substrate proteins, which are then targeted for degradation (for review see 27). Emerging experimental evidence indicates that protein degradation via the Ubi/26S proteasome system plays important roles in plant innate immune response (for review see 28).
In rice, several proteins with activity of E3 Ubi ligases, a group of enzymes required for ubiquitination of substrate proteins in the Ubi/26S proteasome system, are involved in regulating innate immune response 29, 30, 31. For example, SPL1, a U-box protein with E3 Ubi ligase activity, is a negative regulator of cell death and innate immunity against M. oryzae and Xanthomonas oryzae pv. oryzae (Xoo ), the causal agent of bacterial leaf blight disease 30. XB3, an E3 Ubi ligase, is necessary for full accumulation of the XA21 protein and for XA21-mediated innate immune response against Xoo 31. XA21 was recently identified as a PRR with race-specific feature against Xoo 32. We characterized here a rice gene, OsBBI1, which is induced by blast fungus and benzothiadiazole (BTH), and encodes a RING protein with E3 Ubi ligase activity in vitro. Functional analyses using a Tos17-insertion mutant and transgenic overexpression lines demonstrated that OsBBI1 mediates broad-spectrum resistance against multiple races of blast fungus by modifying cell wall defence responses. These findings provide new insights into the cellular and molecular mechanisms of broad-spectrum resistance of rice against blast disease.
Results
OsBBI1 is induced by BTH and M. oryzae
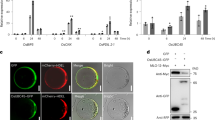
To understand the molecular basis of immune response in rice, we performed microarray gene expression profiling, and identified a group of genes upregulated by M. oryzae and/or Xoo 33. Our analysis indicated that one gene, designated as OsBBI1 (BLAST AND BTH-INDUCED 1, GenBank accession number Os06g03580), was strongly induced by M. oryzae infection, and by SA and BTH, a functional analogue of SA. Expression of OsBBI1 was induced in M. oryzae- infected plants within a period of 48 h, and reached the peak at 24 h after inoculation (Figure 1A). OsBBI1 was upregulated by BTH and SA treatment and reached the peak at 12 h (BTH) or at 24 h (SA) after treatment, respectively; but was not inducible by JA and Xoo infection (Figure 1B, data not shown). The OsBBI1 gene is expressed in root, stem, sheath, and leaf tissues (Supplementary information, Figure S1). These data suggest that OsBBI1 may function in disease resistance to rice blast fungus.
Induction of OsBBI1 in rice by BTH, SA and M. oryzae. (A) Expression of OsBBI1 induced by M. oryzae. (B) Expression of OsBBI1 induced by BTH and SA. Three-week-old rice seedlings were treated by foliar spraying of 300 μM BTH, 1 mM SA, 100 μmol/l JA, or water as a control, inoculated by foliar spraying of spore suspension of M. oryzae (105 spores/ml) or 0.02% Tween 20 in water as mock inoculation. Leaf samples were collected as indicated after treatment or inoculation and expression of OsBBI1 was analysed by RT-PCR for 32 cycles with actin gene as an internal control (26 cycles). JA did not induce OsBBI1. Results are representative of two independent experiments with similar results.
Mutation in OsBBI1 leads to increased susceptibility to M. oryzae
To determine whether OsBBI1 has a function in rice innate immunity, we first examined whether OsBBI1 is required for resistance to blast fungus. A Tos17-insertion mutant line osbbi1 in the Nipponbare mutagenesis resource (ND7061, http://pc7080.abr.affrc.go.jp/∼miyao/pub/tos17/index.html.en) that displays no significant morphological phenotype was identified. The Tos17 element was inserted in the last intron of the OsBBI1 gene (Figure 2A). Reverse transcriptase-polymerase chain reaction (RT-PCR) analyses with a pair of primers amplifying the coding region of the OsBBI1 gene and a pair of primers spanning the Tos17 insertion site showed no detectable transcript of OsBBI1 in osbbi1 plants (Figure 2B). However, the level of OsBBI1 transcript in osbbi1 plants was similar to that in wild-type (WT) plants when using another pair of primers amplifying the truncated N-terminal region of the OsBBI1 transcript (Figure 2B, right). Thus, it is likely that the insertion of the Tos17 element in the OsBBI1 gene results in a “loss-of-function” mutant.
Mutation in OsBBI1 led to enhanced susceptibility to M. oryzae. (A) Genomic structure of the OsBBI1 gene and location of the Tos17 element. Filled boxes indicate exons while black lines indicate introns. The primers are indicated to detect the OsBBI1 transcripts. (B) Detection of the OsBBI1 transcript in osbbi1 mutant. Left, semi-quantitative RT-PCR was performed to detect the full OsBBI1 transcript in osbbi1 and WT plants using a pair of primers (1F/1R) as indicated in A. The actin gene was used as an internal control. Right, quantitative real-time PCR was performed to detect the OsBBI1 transcripts using two pairs of primers (2F/2R and 3F/3R) as indicated in A, respectively. Note that the primers 2F and 2R also detected same levels of the OsBBI1 coding region in osbbi1. (C) Representative disease symptoms on leaves of the osbbi1 and WT plants to different strains (str.), races are indicated. (D) Disease severity on leaves of the osbbi1 and WT plants. Three-week-old plants at the four-leaf stage were inoculated by foliar spraying spore suspension (5 × 105 spores per ml) prepared from different races of M. oryzae and photos were taken at 5 d.p.i. At least 40 plants in each experiment were evaluated for disease scores using an international nine-scale standard. Data presented are the means ± standard errors from three independent experiments. Different letters indicate significant difference at P = 0.05.
We then analysed the disease phenotype of osbbi1 mutant plants on infection by different races of M. oryzae. With seedling inoculation, the osbbi1 plants showed increased susceptibility to races ZD1 (strain 99-188), ZD3 (strain 98-271), and ZG1 (strain 94-10), resulting in an average of one grade higher disease index than the WT Nipponbare plants (Figure 2C and 2D). Similar results were obtained in detached leaf assays (Supplementary information, Figure S2A). Disease lesions on the osbbi1 leaves were significantly larger than on the WT leaves after inoculation with two races of M. oryzae (Supplementary information, Figure S2B). These results indicate that OsBBI1 is required for full immunity in rice against M. oryzae.
Overexpression of OsBBI1 leads to broad-spectrum resistance against M. oryzae
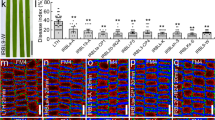
To explore the function of OsBBI1 in rice blast disease resistance, we generated independent OsBBI1-overexpressing transgenic lines (OsBBI1-OE) and compared the disease phenotype of these transgenic lines against M. oryzae. RT-PCR analysis showed that the expression levels of OsBBI1 in representative stable transgenic lines (OE-255, OE-256, and OE-259, T3-T5 generations) were higher than that in the WT TP09 plants (Supplementary information, Figure S3). We used another four different races of M. oryzae, ZE1 (strain 98-48-3), ZA49 (strain 89-151), ZB1 (strain 2000-2), and ZB15 (strain 09-31-1), which are known to be highly virulent on japonica rice. Figure 3 shows that the overall disease scores in the three OsBBI1-OE lines were lower than those in the WT plants (Figure 3A). Disease severities on the OsBBI1-OE plants were on average 1.1–2.5 grades lower than those on the WT TP309 plants (Figure 3B). Similar results were obtained in detached leaf assays (Supplementary information, Figure S4). We compared the disease lesion sizes on the OsBBI1-OE and WT plants after inoculation with traces of ZB1 (strain 2000-2) and ZB15 (strain 09-31-1). As shown in Figure 3C, lesion sizes on the OsBBI1-OE plants were significantly reduced compared with those on the WT plants, resulting in 50-80% and 45-50% reduction when infected by ZB1 and ZB15, respectively. These results confirm that OsBBI1-mediated resistance is efficient against multiple races of blast fungus.
Overexpression of OsBBI1 in transgenic rice confers broad-spectrum resistance against multiple races of M. oryzae. (A) Representative disease symptoms on leaves of the OsBBI1-OE and WT plants. Three-week-old plants were inoculated with different races of M. oryzae and photos were taken at 5 d.p.i. (B) Disease severity on leaves of the OsBBI1-OE and WT plants. At least 40 plants in each experiment were evaluated for disease scores. (C) Lesion sizes on leaves of the OsBBI1-OE and WT plants. At least 100 lesions from 20 representative diseased leaves of 20 plants were measured. Data presented are the means ± standard errors from three independent experiments. Different letters indicate significant difference at P = 0.05 (B, C).
Increased accumulation of H2O2 in OsBBI1 overexpression plants
Since ROS, such as H2O2, are actively involved in defence responses in rice 34, 35, we further analysed the accumulation of H2O2 in sheath epidemic cells at infection sites, using 3,3′-diaminobenzidine (DAB) staining 36. The accumulation of H2O2 was evaluated at 24 h.p.i. and was grouped into five types (A-E) for each infection site (Figure 4A). Most cells at infection sites were not, or only slightly stained in the WT (types A and B), while the percentages of H2O2-stained cells (type C and D) increased in OsBBI1-OE plants (Figure 4B). At 48 h.p.i., the percentages of H2O2-stained epidermal cells (type C + D) were significantly higher in OsBBI1-OE plants than in WT plants (Figure 4B). In contrast, the percentages of H2O2-stained epidermal cells in mutant osbbi1 plants were slightly and significantly lower than those in WT plants at 24 and 48 h.p.i., respectively (Figure 4C). The results indicate that overexpression of OsBBI1 in transgenic plants increases the capacity to prime for accumulation of H2O2 in response to M. oryzae infection.
Accumulation of H2O2 in sheath epidermal cells at infection sites by M. oryzae. Leaf sheaths from 4-week-old plants were inoculated by injection with spore suspension of M. oryzae. (A) Micrographs of the cells with distinct H2O2 accumulation levels in inoculated leaf sheaths stained with DAB at 24 h.p.i. Leaf sheaths from 2-month-old plants were used for experiments. Type A, successful fungal colonization of cells with no DAB staining visible; type B, DAB staining in appressoria site; type C, DAB staining in a primary epidermal cell following fungal invasion; type D, DAB staining a primary epidermal cell and adjacent cells at infection sites; type E, deep DAB staining in a primary epidermal cell and adjacent cells. (B, C) Frequency distribution of the DAB staining cell types at 24 and 48 h.p.i. in sheath epidermal cells of the OsBBI1-OE and WT plants (B) and of the osbbi1 and WT plants (C). Data presented are the mean from three independent experiments with leaf sheaths from five individual plants in each experiment (B, C). At least 100 single-cell infection sites were examined in each experiment. Asterisks above the bars indicate significant difference at P = 0.05.
Enhancement of cell wall defence response in OsBBI1 overexpression plants
The cell wall is the first layer of defence against pathogen infection, and cell wall enhancement during defence is associated with ROS accumulation. Our observations that blast fungus penetrated less into and higher H2O2 accumulation in the OsBBI1-OE cells suggested that cell wall defence might be enhanced in OsBBI1-OE plants, resulting in enhanced blast resistance. To explore this possibility, we analysed the levels of phenolic compounds and cross-linking of wall-associated proteins in sheath epidermal cells at infection sites of M. oryzae by autofluorescence and Coomassie Brilliant Blue staining. Strong autofluorescence and cross-linking of wall-associated proteins were observed at infection sites and adjacent cells of the OsBBI1-OE plants, compared with relatively weak results in WT cells at 24 and 48 h.p.i. (Figure 5A and 5C). The percentages of autofluorescent cell walls and cross-linking increased by ∼10% in the OsBBI1-OE plants compared with that in the WT plants at 24 h.p.i. This increase was much more significant at 48 h.p.i. (Figure 5B and 5D). In contrast, the percentages of autofluorescent cell walls and cross-linking of wall-associated proteins at infection sites were lower in the osbbi1 plants than those in the WT plants at 24 and 48 h.p.i. (Figure 5E and 5F). We further measured the thickness of cell walls in sheath tissues. The cell walls were 25-30% thicker in OsBBI1-OE cells than in WT cells (Figure 6A and 6B). In contrast, the thickness of cell walls in the osbbi1 cells showed a 35% decrease compared with that in the WT cells (Figure 6C and 6D). These data indicated that OsBBI1 mediates defence response by reinforcing cell walls, associated with the increase in ROS accumulation and cross-linking of wall-associated proteins.
Accumulation of phenolic compounds and cross-linking of proteins in cell walls of rice sheath epidermal cells at infection sites. Leaf sheaths from 4-week-old plants were inoculated by injection with spore suspension of M. oryzae. (A) Representative autofluorescence images of sheath epidermal cells at infection sites in OsBBI1-OE and WT TP09 plants. (B, E) Deposition of autofluorescent phenolics in sheath epidermal cells of the OsBBI1-OE and WT plants (B) and of the osbbi1 and WT Nipponbare plants (E). (C) Representative micrographs of cross-linking of wall-associated proteins stained with Coomassie Brilliant Blue dye in sheath epidermal cells at infection sites in the OsBBI1-OE and WT plants. (D, F) Cross-linking of proteins in sheath epidermal cell walls of the OsBBI1-OE and WT plants (D) and of the osbbi1 and WT plants (F). Arrows in A and C indicate fungal appressoria at infection sites. Data presented in B, D, E, and F are the means ± standard errors from three independent experiments with leaf sheaths from five individual plants in each experiment. At least 100 single-cell infection sites were examined in each experiment. Different letters indicate significant difference at P = 0.05.
Comparison of cell wall thickness in OsBBI1-OE, osbbi1 mutant and WT plants. (A, B) Cell walls in OsBBI1-OE and WT Nipponbare plants. (C, D) Cell walls in osbbi1 and WT TP309 plants. Sheath sections from five individual plants were used for observation and at least 20 measurements were carried out for each sheath section. Data presented in B and D are the means ± standard errors. Different letters indicate significant difference at P = 0.05. Bar = 500 nm.
OsBBI1 encodes a RING protein with E3 ligase activity
The OsBBI1 gene encodes a 261-aa protein, which contains a conserved RING domain at the C-terminal end. Seven types of RING domains have been identified, including two canonical RING types, RING-HC (C3HC4) and RING-H2 (C3H2C3), and five modified RING domain types, RING-v, RING-C2, RING-D, RING-S/T, and RING-G 37. The RING domain in OsBBI1 contains the highly conserved C-X2-C-X14-C-X1-H-X2-H-X2-C-X10-C-X2-C structure (Figure 7A), which is commonly present in the RING-H2 type of RING proteins 37. Therefore, we predicted that OsBBI1 belongs to the RING-H2 type of the RING family (Supplementary information, Figure S5). Of these, Arabidopsis BIG BROTHER, SDIR1, SIS3, RING1, NLA, SINAT5, and XBAT32, and rice EL5 and GW2 possess E3 ligase activity in vitro 38, 39, 40, 41, 42, 43, 44, 45, 46. NLA, SIS3, SDIR1, and RING1 play roles in biotic and abiotic stress responses 40, 41, 42, 43. Furthermore, the RING domain and some well-conserved residues within this domain are critical to E3 ligase activity for most RING proteins 37. To explore whether the OsBBI1 protein has E3 Ubi ligase activity, we expressed full-length OsBBI1 as a six His-tagged fusion protein (His-OsBBI1) in Escherichia coli and purified the recombinant protein for enzyme activity assay (Figure 7). To examine the importance of the RING domain in E3 Ubi ligase activity, a truncated mutant of OsBBI1, OsBBI1ΔRING, in which the RING domain was deleted, was also generated as a His-tagged fusion protein, and assayed for E3 ligase activity (Figure 7). In the time-course experiments, clear protein ubiquitination was observed at 1 h after incubating His-OsBBI1 with Ubi, E1, and E2 enzymes in the reactions. The level of protein ubiquitination increased with incubation time, indicating the E3 ligase activity of His-OsBBI1 (Figure 7B). In the presence of Ubi, E1, and E2 enzymes, His-OsBBI1 could carry out self-ubiquitination, while no clear protein ubiquitination was detected in the absence of E1, E2, or His-OsBBI1 (Figure 7C). The truncated mutant, OsBBI1ΔRING, did not show E3 ligase activity in the reactions (Figure 7C). These results show that OsBBI1 is a functional E3 ligase, and the RING domain in OsBBI1 is essential to its E3 Ubi ligase activity.
OsBBI1 is a RING-H2 protein with E3 ligase activity in vitro. (A) Amino acid alignments of the OsBBI1 RING domain with RING domains of other proteins from Arabidopsis (At), rice (Os) and maize (Zm). Filled triangles indicate the conserved cysteine (C) residues, while asterisks indicate conserved histidine (H) residues. (B, C) E3 ligase activity of OsBBI1 in vitro. His-tagged OsBBI1 or OsBBI1ΔRING fusion protein was assayed for E3 ubiquitin ligase activity in the presence of E1, E2, and Ubi. Ubiquitinated proteins were detected by protein blot analysis using an antibody to Ubi, with time-course increase in ubiquitinated proteins shown in B. Arrow indicates the ubiquitinated proteins.
Discussion
Blast disease is the most economically devastating disease in rice, and blast resistance is a prerequisite for commercial variety registration and release in China. However, blast resistance can be rapidly broken down due to the race diversity of M. oryzae 47. Thus, breeding rice for broad-spectrum blast resistance has been a long-term task for rice breeders, with limited success due to the availability of resistance genes and our limited knowledge of resistance mechanisms. This study shows that a blast fungus- and BTH-induced gene OsBBI1, encoding a RING protein with E3 ligase activity, plays a role in basal resistance to blast fungus. Loss of OsBBI1 function resulted in increased susceptibility, whereas overexpression of OsBBI1 in transgenic plants led to enhanced resistance against multiple races of M. oryzae (Figures 2 and 3), indicating that OsBBI1 confers broad-spectrum resistance in rice against blast disease.
Several E3 ligases in rice have been reported to be involved in innate immunity 29, 30, 31. However, OsBBI1 is distinct from the E3 ligases previously reported to be involved in rice innate immunity in terms of structural characteristics and biological functions. Structurally, OsBBI1 belongs to the RING-H2 type of RING proteins, and only contains a conserved RING domain at its C-terminus. SPL1, a U-box protein with E3 Ubi ligase activity, contains both a U-box domain and an armadillo repeat domain, while XB3, an XA21-binding protein with E3 Ubi ligase activity, has an ankyrin repeat domain in its N-terminus in addition to a RING domain at its C-terminus 30, 31. Functionally, experimental data presented in this study demonstrate that OsBBI1 positively regulates rice defence response against M. oryzae, but not against Xoo (data not shown). SPL1 negatively regulates cell death and innate immunity against both M. oryzae and Xoo 30, while XB3 seems to have a specific function in XA21-mediated innate immune response against Xoo 31. Therefore, this study adds OsBBI1, representing a novel E3 Ubi ligase, to the list of proteins with E3 ligase activity involved in rice innate immunity.
Broad-spectrum resistance is generally referred to as resistance to majority of geographically different isolates of the same pathogen and/or resistance to two or more unrelated pathogens. We did not observe the involvement of OsBBI1 in disease resistance against bacterial blight, indicating that OsBBI1-mediated resistance is likely specific to defence against blast fungus, consistent with its induction by BTH and M. oryzae but not by Xoo. In rice, several R genes/loci such as Pi5, Pi9, Pi21, Pi33, Pi39(t), and Pi40(t), have been identified to confer broad-spectrum blast resistance 9, 10, 48, 49, 50, 51]. However, molecular mechanisms by which these R genes provide broad-spectrum resistance remain elusive. The characterization of OsBBI1 as a novel component in rice immunity against M. oryzae may provide an additional approach to dissect the molecular and biochemical mechanisms downstream of pathogen recognition. Furthermore, OsBBI1 may provide a practical tool for engineering broad-spectrum blast resistance in rice breeding.
R gene-mediated resistance often provides full race-specific protection. In contrast, resistance controlled by quantitative trait loci (QTLs) is non-race-specific but partial (for review see 7). Compared with R gene-mediated high level of disease resistance, overexpression of OsBBI1 only conferred a relatively low level of resistance and mutation in the OsBBI1 gene resulted in partial loss of resistance against multiple races of M. oryzae (Figures 2 and 3). This indicates that OsBBI1 may mediate non-race-specific basal resistance against M. oryzae in rice, and is probably involved in an unrecognized PTI network. Some blast resistance QTLs have been identified in rice and some have been characterized 7, 10, 26. The OsBBI1 gene (Os06g03580) is located in a region of chromosome 6, in which a number of blast resistance QTLs have been identified 52, 53, 54, 55. The features of OsBBI1-mediated basal and partial resistance suggest that OsBBI1 might be a QTL against M. oryzae, such as other rice defence genes 56.
Extensive studies have indicated that ROS is an important component of plant defence responses associated with cell wall reinforcement 57, 58. The invasion of M. oryzae hyphae often stimulates rapid production of H2O2 in rice cells at infection sites as an early defence response 59. Small GTPase Rac complex-mediated accumulation of ROS generated through NADPH oxidases is required for innate immune response against M. oryzae 11, 12, 13, 14, 15. Most recently, Chi et al. 60 reported that virulent M. oryzae strains have developed ROS-scavenging ability through the DES1 protein, thus suppressing ROS-mediated innate immune response in host cells. OsBBI1-OE plants accumulated higher, while osbbi1 mutant plants generated lower levels of H2O2 than WT plants in cells at the sites of M. oryzae infection (Figure 4). This correlates with increased levels of autofluorescence and cross-linking of proteins in cell walls in the OsBBI1-OE plants. However, overexpression of OsBBI1 does not result in constitutive accumulation of H2O2, autofluorescent materials, and cross-linking of cell wall proteins because the levels of H2O2, autofluorescence, and cross-linking of proteins in the walls of cells beyond the infection sites were similar with OsBBI1-OE and WT plants. Thus, the OsBBI1-mediated ROS generation and cell wall defences can only be activated in OsBBI1-OE plants on infection of M. oryzae. This can be explained by an unrecognized mechanism, in which OsBBI1 may target the degradation of negative regulator(s) of defence responses, which is probably induced after pathogen infection. OsBBI1 could also target the degradation of suppressor of host defence responses, which is secreted by M. oryzae during infection.
Recent studies demonstrated the importance of the cell wall in immune responses against diverse pathogens 61, 62, 63, 64, 65. Thickness or reinforcement of the cell wall plays important roles in counteracting the penetration of fungal pathogens 66, 67. Consistent with ROS accumulation and cross-linking of cell wall proteins, OsBBI1-OE plants develop thicker cell walls, while the osbbi1 mutant generates thinner walls compared with corresponding WT plants (Figure 6). This indicates that functions of OsBBI1 in cell wall defence responses are likely executed via regulating ROS production and/or the cell wall synthesis pathway. Further identification of the OsBBI1 target (s) will provide deeper insights into the biochemical and molecular basis of OsBBI1-mediated immunity in rice.
Materials and Methods
Growth of rice plants
Rice (Oryza sativa L.) subsp. indica cv. Yuanfengzao, japonica cv. Taipei 309 (TP309), and Nipponbare were used in this study. Transgenic plants were selected by GUS staining and PCR detection. All rice seedlings were grown in a growth room at 25 °C with 14 h light/10 h darkness. Three-week-old seedlings were used for blast disease assays and other experiments.
Generation and characterization of overexpression and osbbi1 mutant plants
A Tos17 insertion line of the OsBBI1 gene, ND7061, was obtained from the Tos17 insertion resource (http://pc7080.abr.affrc.go.jp/∼miyao/pub/tos17/index.html.en). PCR-based genotyping was performed to screen for homozygous plants. Genotyping was performed using OsBBI1-specific primers, OsBBI1-g-1F (5′-CTCCTCACACGCTAGGAAGG-3′) and OsBBI1-g-1R (5′-GTCTATCCGCTTTCAGACGC-3′), along with a Tos17-specific primer Tos17-1R (5′-ATTGTTAGGTTGCAAGTTAGTTAAGA-3′). Homozygous plants were used for studies. The plasmid for overexpression was generated by cloning the full-length coding region (1.3 kb) of OsBBI1 into the rice expression vector 35S-C1301, and was transformed into cv. TP309 (japonica) to generate more than 20 independent lines. Stable lines were assayed for disease resistance with the continuous generations (T1 to T3).
Treatment of chemical inducers
Seedlings of cv. Yuanfengzao (indica) were sprayed with BTH (0.3 mM), SA (1.5 mM, pH 6.5), and JA (0.1 mM) solutions, and with distiled water containing 0.05% Tween 20 as a control. Leaf samples were collected within a period and stored at −80 °C until use.
Inoculation and disease assays
Different strains of M. oryzae, belonging to races of ZA49 (strain 89-151), ZD3 (strain 98-271), ZG1 (strain 94-10), ZE1 (strain 98-48-3), ZD1 (strain 99-188), ZB1 (strain 2000-2), and ZB15 (strain 09-31-1) were used for blast resistance assay. Three-week-old seedlings were spray-inoculated with spores (5 × 105 per ml with 0.05% Tween-20 in water) as described 68. Disease severity was evaluated using a standard international 0-9 scale (0 = resistant and 9 = susceptible) at 6 d.p.i. Leaf blast spot inoculation was performed as described 16. To observe cellular defence, a sheath inoculation method was used as described 69. For microscope observation, sheath sections were sampled at 24 and 48 h.p.i. At least five sections were observed for each sample.
Visualization of cytological responses
To detect H2O2 accumulation at infection sites, the sheath sections were vacuum infiltrated in DAB-HCl solution (1 mg/ml, pH 3.8) 36 for 30 min and incubated in the growth chamber for 8 h. Trimmed sheath segments were mounted in 50% glycerol and examined for the formation of brown-red precipitate under bright-field with a Leica CTR5000 microscopy (Leica Microsystems, Hong Kong, China). To detect phenolic compounds, sheath segments were stained for 30 min in a solution containing 0.01% (w/v) aniline blue and 0.15 M K2HPO4. They were visualized as autofluorescence under blue light epifluorescence with a Leica TCS SP5 fluorescent microscopy (GPF filter set; excitation at 405 nm, dichroic beamsplitter of 500-550 nm; Leica Microsystems, Hong Kong, China) according to the method described previously 59. To detect protein cross-linking, sheath segments were submerged in 1% sodium dodecyl sulphate (SDS) for 24 h at 80 °C and stained in 0.1% Coomassie Brilliant Blue in a solution of 40% ethanol and 10% acetic acid for 15 min. They were subsequently rinsed in a solution of 40% ethanol and 10% acetic acid, followed by mounting in 50% glycerol and examination using a Leica CTR5000 microscopy (Leica Microsystems, Hong Kong, China).
Transmission electron microscopy (TEM)
Sheath segments from 2-month-old rice plants were fixed with 2.5% glutaraldehyde in phosphate buffer (100 mmol/l, pH7.0) for at least 4 h, washed three times with the same phosphate buffer for 15 min each, post fixed with 1% osmium tetroxide in the phosphate buffer for 1 h, and washed three times with the phosphate buffer. The sheath segments were then embedded in Epon 812, and ultra-thin sections were stained by uranyl acetate and alkaline lead citrate for 15 min, respectively, and observed in TEM of Model H-7650 (Hitachi, Tokyo, Japan).
E3 Ubi ligase activity assay
The coding sequence of the OsBBI1 gene was cloned into pET-32 vector at BamHI and HindIII sites, and the recombinant plasmid was introduced into the E. coli strain (DE3). To generate the truncated mutant OsBBI1ΔRING, the coding sequence was amplified using a pair of primers, OsBBI1-dRING-1F (5′-GCGGGATCCATGGCCACCGTGGGGCAGCCT-3′ and OsBBI1-dRING-1R (5′-GCGAAGCTTCTAATCATGGTTTGCCTTCCTTG-3′, and cloned into pET-32 vector at BamHI and HindIII sites. Expression of OsBBI1 and OsBBI1ΔRING fusion proteins in E. coli cells was induced by 1 mmol/l isopropyl-D-thiogalactoside at 37 °C for 4 h. The His-tagged OsBBI1 fusion protein was purified using a His-Bind Kit following the manufacturer's protocols (NovaGen, Madison, WI, USA). Assays for in vitro ubiquitination were carried out as described previously, with slight modifications 38. In brief, 0.1 μg human E1 (Merk BioSciences, Nottingham, UK), 0.22 μg human E2 (UbcH5b) (Merk BioSciences, Nottingham, UK), 10 μg Ubi, and 1 μg purified His-tagged OsBBI1 or OsBBI1ΔRING fusion protein were incubated in a 30 μl reaction mix (50 mM Tris-HCl, 5 mM MgCl2, 2 mM ATP and 0.5 mM DTT, pH 7.5) at 30 °C for 0-3 h. The reaction was stopped with 1× SDS-polyacrylamide gel electrophoresis (PAGE) loading buffer and boiled in water for 5 min. In vitro ubiquitination of the samples were analysed by SDS-PAGE and polyubiquitinated proteins were detected by protein blotting using an anti-Ubi antibody (Merk BioSciences, Nottingham, UK).
Analysis of gene expression
Total RNA was prepared from the samples using the TRIZOL reagent according to the manufacturer's procedure (Invitrogen, Shanghai, China) and treated with DNase RQ1 (Promega, Madion, WI, USA). First-strand cDNA was synthesized from 600 ng of total RNA using superscript III RT (Invitrogen, Shanghai, China). Semi-quantitative RT-PCR was performed for 28-32 cycles based on the abundance of transcripts of the genes analysed. Primers used in this study are OsBBI1-rt-1F (5′-AGGCAACAACGAAGAGGCTGC-3′) and OsBBI1-rt-1R (5′-TAGTTGGTCAGCCTTGTTGATC-3′) for OsBBI1 expression and detection of OsBBI1 transcript in osbbi1 mutant, and OsActin-1F (5′-TATGGTCAAGGCTGGGTTCG-3′) and OsActin-1R (5′-CCATGCTCGATGGGGTACTT-3′) for actin expression as an internal control. Quantitative real-time PCR was carried out using SYBR premix Ex Taq (TaKaRa, Dalian, China) in a CFX96 Real-Time System (Bio-Rad, Hercules, CA, USA) with 100 ng cDNA and 7.5 pmol of each gene-specific primer. Data were collected from two independent biological samples in triplicate. Expression ratios were calculated using the 2−ΔΔCt method with the elongation factor 1α as reference gene. Relative expression levels were compared with the expression levels before treatment as 1.0. Gene-specific primers used are as follows: OsBBI1-rt-2F, 5′-GAAACACTGCTCTTGCCCAATCTG-3′; OsBBI1-rt-2R, 5′-TGCCTAGCGAACACCAAGTCATAC-3′; OsBBI1-rt-3F, 5′-AGACGCCGAGAAACACTCATTACA-3′; OsBBI1-rt-3R, 5′-CAGCCTTGTTGATCGCCTCCTC-3′; OsEF1a-rt-1F, 5′-GTCATTGGCCACGTCGACTC-3′; OsEF1a-1R, 5′-TGTTCATCTCAGCGGCTTCC-3′.
Statistical analysis
All experiments were repeated independently at least three times. Data collected were statistically analysed by one-way analysis of variance followed by least significant difference test at P = 0.05 using DPS software (http://www.chinadps.net/index.htm).
Accession Number
Sequence data from this article can be found in GenBank under the following accession number: OSBBI1 genomic DNA and proteins (Os06g03580).
Accession codes
References
Jones JD, Dangl JL . The plant immune system. Nature 2006; 444:323–329.
Chisholm ST, Coaker G, Day B, Staskawicz BJ . Host-microbe interactions: shaping the evolution of the plant immune response. Cell 2006; 124:803–814.
Boller T, He SY . Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science 2009; 324:742–744.
Boller T, Felix G . A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 2009; 60:379–406.
Bent AF, Mackey D . Elicitors, effectors, and R genes: the new paradigm and a lifetime supply of questions. Annu Rev Phytopathol 2007; 45:399–436.
Stulemeijer IJ, Joosten MH . Post-translational modification of host proteins in pathogen-triggered defence signalling in plants. Mol Plant Pathol 2008; 9:545–60.
Liu J, Wang X, Mitchell T, et al. Recent progress and understanding of the molecular mechanisms of the rice-Magnaporthe oryzae interaction. Mol Plant Pathol 2010; 11:419–427.
Jia Y, McAdams SA, Bryan GT, Hershey HP, Valent B . Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J 2000; 19:4004–4014.
Qu S, Liu G, Zhou B, et al. The broad-spectrum blast resistance gene Pi9 encodes a nucleotide binding site-leucine-rich repeat protein and is a member of a multigene family in rice. Genetics 2006; 172:1901–1914.
Fukuoka S, Saka N, Koga H, et al. Loss of function of a proline-containing protein confers durable disease resistance in rice. Science 2009; 325:998–1001.
Ono E, Wong HL, Kawasaki T, Hasegawa M, Kodama O, Shimamoto K . Essential role of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci USA 2001; 98:759–764.
Suharsono U, Fujisawa Y, Kawasaki T, Iwasaki Y, Satoh H, Shimamoto K . The heterotrimeric G protein alpha subunit acts upstream of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci USA 2002; 99:13307–13312.
Kawasaki T, Koita H, Nakatsubo T, et al. Cinnamoyl-CoA reductase, a key enzyme in lignin biosynthesis, is an effector of small GTPase Rac in defense signaling in rice. Proc Natl Acad Sci USA 2006; 103:230–235.
Wong HL, Pinontoan R, Hayashi K, et al. Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell 2007; 19:4022–4034.
Thao NP, Chen L, Nakashima A, et al. RAR1 and HSP90 form a complex with Rac/Rop GTPase and function in innate-immune responses in rice. Plant Cell 2007; 19:4035–4045.
Wang Y, Gao M, Li Q, et al. OsRAR1 and OsSGT1 physically interact and function in rice basal disease resistance. Mol Plant-Microbe Interact 2008; 21:294–303.
Nakashima A, Chen L, Thao NP, et al. RACK1 functions in rice innate immunity by interacting with the Rac1 immune complex. Plant Cell 2008; 20:2265–2279.
Chern MS, Fitzgerald HA, Yadav RC, Canlas PE, Dong X, Ronald PC . Evidence for a disease-resistance pathway in rice similar to the NPR1-mediated signaling pathway in Arabidopsis. Plant J 2001; 27:101–113.
Chern M, Fitzgerald HA, Canlas PE, Navarre DA, Ronald PC . Overexpression of a rice NPR1 homolog leads to constitutive activation of defense response and hypersensitivity to light. Mol Plant-Microbe Interact 2005; 18:511–520.
Shimono M, Sugano S, Nakayama A, et al. Rice WRKY45 plays a crucial role in benzothiadiazole-inducible blast resistance. Plant Cell 2007; 19:2064–2076.
Xiong L, Yang Y . Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid-inducible mitogen-activated protein kinase. Plant Cell 2003; 15:745–759.
Yuan Y, Zhong S, Li Q, et al. Functional analysis of rice NPR1-like genes reveals that OsNPR1/NH1 is the rice orthologue conferring disease resistance with enhanced herbivore susceptibility. Plant Biotechnol J 2007; 5:313–324.
Kawano Y, Akamatsu A, Hayashi K, et al. Activation of a Rac GTPase by the NLR family disease resistance protein Pit plays a critical role in rice innate immunity. Cell Host Microbe 2010; 7:362–375.
Chen L, Hamada S, Fujiwara M, et al. The Hop/Sti1-Hsp90 chaperone complex facilitates the maturation and transport of a PAMP receptor in rice innate immunity. Cell Host Microbe 2010; 7:185–196.
Yang Y, Qi M, Mei C . Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant J 2004; 40:909–919.
Manosalva PM, Davidson RM, Liu B, et al. A germin-like protein gene family functions as a complex quantitative trait locus conferring broad-spectrum disease resistance in rice. Plant Physiol 2009; 149:286–296.
Smalle J, Vierstra RD . The ubiquitin 26s proteasome proteolytic pathway. Annu Rev Plant Biol 2004; 55:555–590.
Zeng L, Vega-Sanchez ME, Zhu T, Wang G . Ubiquitination-mediated protein degradation and modification: an emerging theme in plant-microbe interactions. Cell Res 2006; 16:413–426.
Takai R, Matsuda N, Nakano A, et al. EL5, a rice N-acetylchitooligosaccharide elicitor-responsive RING-H2 finger protein, is a ubiquitin ligase which functions in vitro in co-operation with an elicitor-responsive ubiquitin-conjugating enzyme, OsUBC5b. Plant J 2002; 30:447–455.
Zeng L, Qu S, Bordeos A, et al. Spotted leaf11, a negative regulator of plant cell death and defense, encodes a U-box/armadillo repeat protein endowed with E3 ubiquitin ligase activity. Plant Cell 2004; 16:2795–2808.
Wang Y, Pi L, Chen X, et al. Rice XA21 binding protein 3 is a ubiquitin ligase required for full Xa21-mediated disease resistance. Plant Cell 2006; 18:3635–3646.
Lee S, Han S, Sririyanum M, Park C, Seo Y, Ronald PC . A type I-secreted, sulfated peptide triggers XA21-mediated innate immunity. Science 2009; 326:850–853.
Li Q, Chen F, Sun L, Zhang Z, Yang Y, He Z . Expression profiling of rice genes in early defense responses to blast and bacterial blight pathogens using cDNA microarray. Physiol Mol Plant Pathol 2006; 68:51–60.
He Z, Wang Z, Li J, et al. Perception of brassinosteroids by the extracellular domain of the receptor kinase, BRI1. Science 2000; 288:2360–2363.
Zhang H, Zhang X, Mao B, Li Q, He Z . Alpha-picolinic acid, a fungal toxin and mammal apoptosis-inducing agent, elicits hypersensitive-like response and enhances disease resistance in rice. Cell Res 2004; 14:27–33.
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB . Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 1997; 11:1187–1194.
Stone SL, Hauksdottir H, Troy A, Herschleb J, Kraft E, Callis J . Functional analysis of the RING-type ubiquitin ligase family of Arabidopsis. Plant Physiol 2005; 137:13–30.
Song X, Huang W, Shi M, Zhu MZ, Lin H . A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat Genet 2007; 39:623–630.
Disch S, Anastasiou E, Sharma VK, Laux T, Fletcher JC, Lenhard M . The E3 ubiquitin ligase BIG BROTHER controls Arabidopsis organ size in a dosage-dependent manner. Curr Biol 2006; 16:272–279.
Zhang Y, Yang C, Li Y, et al. SDIR1 is a RING finger E3 ligase that positively regulates stress-responsive abscisic acid signaling in Arabidopsis. Plant Cell 2007; 19:1912–1929.
Huang Y, Li CY, Pattison DL, Gray WM, Park S, Gibson SI . SUGAR-INSENSITIVE3, a RING E3 ligase, is a new player in plant sugar response. Plant Physiol 2010; 152:1889–1900.
Lin S, Martin R, Mongrand S, et al. RING1 E3 ligase localizes to plasma membrane lipid rafts to trigger FB1-induced programmed cell death in Arabidopsis. Plant J 2008; 56:550–561.
Yaeno T, Iba K . BAH1/NLA, a RING-type ubiquitin E3 ligase, regulates the accumulation of salicylic acid and immune responses to Pseudomonas syringae DC3000. Plant Physiol 2008; 148:1032–1041.
Xie Q, Guo HS, Dallman G, Fang S, Weissman AM, Chua NH . SINAT5 promotes ubiquitin-related degradation of NAC1 to attenuate auxin signals. Nature 2002; 419:167–170.
Takai R, Matsuda N, Nakano A, et al. EL5, a rice N-acetylchitooligosaccharide elicitor-responsive RING-H2 finger protein, is a ubiquitin ligase which functions in vitro in co-operation with an elicitor-responsive ubiquitin-conjugating enzyme, OsUBC5b. Plant J 2002; 30:447–455.
Nodzon LA, Xu W, Wang Y, Pi L, Chakrabarty PK, Song W . The ubiquitin ligase XBAT32 regulates lateral root development in Arabidopsis. Plant J 2004; 40:996–1006.
Wilson RA, Talbot NJ . Under pressure: investigating the biology of plant infection by Magnaporthe oryzae. Nat Rev Microbiol 2009; 7:185–195.
Lee SK, Song MY, Seo YS, et al. Rice Pi5-mediated resistance to Magnaporthe oryzae requires the presence of two coiled-coil-nucleotide-binding-leucine-rich repeat genes. Genetics 2009; 181:1627–1638.
Jeung JU, Kim BR, Cho YC, et al. A novel gene, Pi40(t), linked to the DNA markers derived from NBS-LRR motifs confers broad spectrum of blast resistance in rice. Theor Appl Genet 2007; 115:1163–1177.
Liu X, Yang Q, Lin F, et al. Identification and fine mapping of Pi39(t), a major gene conferring the broad-spectrum resistance to Magnaporthe oryzae. Mol Genet Genomics 2007; 278:403–410.
Liu G, Lu G, Zeng L, Wang G . Two broad-spectrum blast resistance genes, Pi9(t) and Pi2(t), are physically linked on rice chromosome 6. Mol Genet Genomics 2002; 267:472–480.
Ballini E, Morel JB, Droc G, et al. A genome-wide meta-analysis of rice blast resistance genes and quantitative trait loci provides new insights into partial and complete resistance. Mol Plant-Microbe Interact 2008; 21:859–868.
Xu J, Wang J, Ling Z, Zhu L . Analysis of rice blast resistance genes by QTL mapping. Chin Sci Bull 2004; 49:337–342.
Wu J, Fan Y, Li D, Zheng K, Leung H, Zhuang J . Genetic control of rice blast resistance in the durably resistant cultivar Gumei 2 against multiple isolates. Theor Appl Genet 2005; 111:50–56.
Tabien E, Li Z, Paterson H, Marchetti A, Stansel W, Pinson M . Mapping QTLs for field resistance to the rice blast pathogen and evaluating their individual and combined utility in improved varieties. Theor Appl Genet 2002; 105:313–324.
Hu K, Qiu D, Shen X, Li X, Wang S . Isolation and manipulation of quantitative trait loci for disease resistance in rice using a candidate gene approach. Mol Plant 2008; 1:786–793.
Grant JJ, Loake GJ . Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol 2000; 124:21–29.
Torres MA, Jones JD, Dangl JL . Reactive oxygen species signaling in response to pathogens. Plant Physiol 2006; 141:373–378.
De Vleesschauwer D, Djavaheri M, Bakker PA, Hofte M . Pseudomonas fluorescens WCS374r-induced systemic resistance in rice against Magnaporthe oryzae is based on pseudobactin-mediated priming for a salicylic acid-repressible multifaceted defense response. Plant Physiol 2008; 148:1996–2012.
Chi MH, Park SY, Kim S, Lee YH . A novel pathogenicity gene is required in the rice blast fungus to suppress the basal defenses of the host. PLoS Pathog 2009; 5:e1000401.
Vogel JP, Raab TK, Schiff C, Somerville SC . PMR6, a pectate lyase-like gene required for powdery mildew susceptibility in Arabidopsis. Plant Cell 2002; 14:2095–2106.
Vogel JP, Raab TK, Somerville CR, Somerville SC . Mutations in PMR5 result in powdery mildew resistance and altered cell wall composition. Plant J 2004; 40:968–978.
Collins NC, Thordal-Christensen H, Lipka V, et al. SNARE-protein-mediated disease resistance at the plant cell wall. Nature 2003; 425:973–977.
Hernandez-Blanco C, Feng D, Hu J, et al. Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell 2007; 19:890–903.
Sanchez-Rodriguez C, Estevez JM, Llorente F, et al. The ERECTA receptor-like kinase regulates cell wall-mediated resistance to pathogens in Arabidopsis thaliana. Mol Plant-Microbe Interact 2009; 22:953–963.
Somerville C, Bauer S, Brininstool G, et al. Toward a systems approach to understanding plant cell walls. Science 2004; 306:2206–2211.
Schulze-Lefert P . Knocking on the heaven's wall: pathogenesis of and resistance to biotrophic fungi at the cell wall. Curr Opin Plant Biol 2004; 7:377–383.
Deng Y, Zhu X, Shen Y, He Z . Genetic characterization and fine mapping of the blast resistance locus Pigm(t) tightly linked to Pi2 and Pi9 in a broad-spectrum resistant Chinese variety. Theor Appl Genet 2006; 113:705–713.
Koga H, Dohi K, Nakayachi O, Mori M . A novel inoculation method of Magnaporthe grisea for cytological observation of the infection process using intact leaf sheaths of rice plants. Physiol Mol Plant Pathol 2004; 64:67–72.
Acknowledgements
We thank the National Institute of Agrobiological Sciences, Japan for providing the Tos17 insertion line and Mr Rongyao Chai, Zhejiang Academy of Agricultural Science for his help in the disease assays. This work was supported by grants from the National Natural Science Foundation of China (30730064 to ZH), the National Key Basic Research and Development Program (2006CB101905 to FS) and the Ministry of Agriculture of China (2008ZX08009-003-1 to ZH and 2009ZX08001-017B to FS).
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary Information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Expression patterns of OsBBI1 in different tissues of rice plants. (PDF 46 kb)
Supplementary information, Figure S2
Increased susceptibility of osbbi1 plants to M. oryzae in detached leaf assays. (PDF 102 kb)
Supplementary information, Figure S3
Expression levels of OsBBI1 in transgenic lines. (PDF 41 kb)
Supplementary information, Figure S4
Increased resistance of the OsBBI1-OE plants to M. oryzae in detached leaf assay. (PDF 113 kb)
Supplementary information, Figure S5
Phylogenetic tree of OsBBI1 and other homologous plant RING proteins. (PDF 89 kb)
Rights and permissions
About this article
Cite this article
Li, W., Zhong, S., Li, G. et al. Rice RING protein OsBBI1 with E3 ligase activity confers broad-spectrum resistance against Magnaporthe oryzae by modifying the cell wall defence. Cell Res 21, 835–848 (2011). https://doi.org/10.1038/cr.2011.4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2011.4
Keywords
This article is cited by
-
Ubiquitination in plant biotic and abiotic stress
Plant Growth Regulation (2023)
-
SCL, Encoding a Chloroplast Signal Recognition Particle Receptor, Affects Chlorophyll Synthesis and Chloroplast Development in Rice
Journal of Plant Growth Regulation (2023)
-
Rice functional genomics: decades’ efforts and roads ahead
Science China Life Sciences (2022)
-
Identification and validation of a key genomic region on chromosome 6 for resistance to Fusarium stalk rot in tropical maize
Theoretical and Applied Genetics (2022)
-
TaClpS1, negatively regulates wheat resistance against Puccinia striiformis f. sp. tritici
BMC Plant Biology (2020)