Abstract
Potassium transporters play crucial roles in K+ uptake and translocation in plants. However, so far little is known about the regulatory mechanism of potassium transporters. Here, we show that a Shaker-like potassium channel AtKC1, encoded by the AtLKT1 gene cloned from the Arabidopsis thaliana low-K+ (LK)-tolerant mutant Atlkt1, significantly regulates AKT1-mediated K+ uptake under LK conditions. Under LK conditions, the Atkc1 mutants maintained their root growth, whereas wild-type plants stopped their root growth. Lesion of AtKC1 significantly enhanced the tolerance of the Atkc1 mutants to LK stress and markedly increased K+ uptake and K+ accumulation in the Atkc1-mutant roots under LK conditions. Electrophysiological results showed that AtKC1 inhibited the AKT1-mediated inward K+ currents and negatively shifted the voltage dependence of AKT1 channels. These results demonstrate that the 'silent' K+ channel α-subunit AtKC1 negatively regulates the AKT1-mediated K+ uptake in Arabidopsis roots and consequently alters the ratio of root-to-shoot under LK stress conditions.
Similar content being viewed by others
Introduction
Potassium is an essential mineral element for plant growth and development, and it plays essential roles in many important physiological and biochemical processes in living plant cells, such as regulation of enzyme activation, electrical neutralization, osmoregulation, control of membrane potential, co-transport of sugars, and so on 1, 2. Plant growth and development need millimolar K+ in the soil or growth medium, but typical K+ concentration at the interface of roots and soils is within micromolar range 3. Thus, plants often encounter low-K+ (LK) stress under natural conditions. Although different plants or different genotypes of a plant species show varied K+ utilization efficiency 4, most plants show K+-deficient symptom under LK stress, typically leaf chlorosis and subsequent inhibition of plant growth and development 5.
Absorption of K+ by plant cells and K+ translocation between different tissues and organs in plants are mediated by plant K+ transporters and channels 2, 6, 7. Over the past decade, a large number of genes encoding plant K+ transporters and channels, particularly for Arabidopsis, have been characterized 6, 7, 8. These K+ transporters vary in K+ affinity, kinetics, transcriptional modulation, regulatory mechanism, etc 2, 6, 7, 8, and they compose a complex system for plant K+ uptake and translocation. Among these K+ transporters and channels, members of the Shaker K+ channel family are well characterized for their potential functions and are probably the most important for K+ uptake and transport in Arabidopsis 8. Most members in this family have been functionally characterized except AKT5 and AtKC1 2, 6, 8. However, little is known about the regulatory mechanism of these K+ transporters. AKT1 has been identified as an important dual-affinity inward-rectifying K+ channel participating in K+ uptake from soil into cells in Arabidopsis roots 9, 10, 11, 12. More recently, we have demonstrated that AKT1 activity is positively regulated by a protein kinase AtCIPK23, and AtCIPK23 is in turn activated by AtCBL1 or AtCBL9 13.
Recent reports showed that channel heterotetramerization is a key regulatory mechanism for K+ channels, which was found not only in animals 14, 15, 16 but also in plants 17, 18. Different kinds of potassium channel subunits may assemble together to form functional heteromeric potassium channels. This heterotetramerization could contribute to regulation of K+ channel activities, as well as generate novel K+ channels with diverse functional properties in activation kinetics, voltage dependence, ionic sensitivity, etc 6, 8, 17. Therefore, plants could employ various heteromeric K+ channels involved in K+ uptake and translocation to meet different physiological requirements 6, 8, 17. Heterotetramerization of plant Shaker K+ channels was initially observed in the Xenopus oocyte heterologous expression system. Dreyer et al. 17 reported that plant K+ channel α-subunits from different tissues, species, and plant families assembled indiscriminately and formed heterotetramers with novel channel properties. The more recent studies using electrophysiological technique showed that Arabidopsis Shaker K+ channel α-subunit AtKC1 could not form functional K+ channel solely, but it may assemble with AKT1, forming functional heterotetrameric potassium channel, and change the channel properties of AKT1 19, 20, 21. However, the comprehensive physiological function of AtKC1 in Arabidopsis K+ acquisition remains unclear.
Here, we report the evidence that AtKC1 regulates AKT1 activity in K+ uptake by Arabidopsis root under LK conditions. Starting from the Arabidopsis thaliana LK-tolerant mutant Atlkt1, we identified that AtKC1, encoded by the AtLKT1 gene, significantly regulates AKT1-mediated K+-uptake under LK conditions. Lesion of AtKC1 significantly enhanced the tolerance of Atkc1 mutants to LK stress and markedly increased K+ uptake and K+ accumulation in Atkc1-mutant roots under LK conditions, while overexpression of AtKC1 resulted in K+ uptake inhibition. The results of voltage-clamp recording using the Xenopus oocyte expression system and patch-clamp recording from root cell protoplasts showed that AKT1-mediated inward K+ currents were significantly inhibited by AtKC1. Our results demonstrate that AtKC1 functions as a negative regulator of AKT1 and plays important roles in AKT1-mediated root K+ uptake and biomass allocation in Arabidopsis, especially under K+-deficient conditions.
Results
Isolation of Arabidopsis LK-tolerant mutant Atlkt1
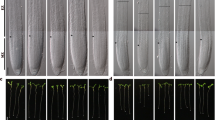
Arabidopsis LK-tolerant (Atlkt) mutants were isolated from EMS-mutagenized M2 seedlings. Among several putative M3 mutants, Atlkt1 mutants showed significant LK-tolerant phenotype, that is, their roots kept growing after being transferred to LK (100 μM) medium, while the roots of wild-type plants stopped growth (Figure 1A). Genetic analysis showed that Atlkt1 harbored monogenic recessive mutation in a nuclear gene (data not shown). Analysis of the F2 generation from a cross between Atlkt1 and wild type revealed a 3:1 segregation ratio of wild type over Atlkt1 (data not shown).
Isolation and phenotype analysis of Atlkt1 (low-K+ tolerant) mutants and map-based cloning of the AtLKT1 gene. (A) Phenotype comparison between wild-type Arabidopsis (Col) and Atlkt1 mutants after they are grown on MS and LK (100 μM K+) mediums for 4 days. (B) Map-based cloning of AtLKT1. The AtLKT1 gene was identified to encode AtKC1. In AtKC1 structure, the filled boxes represent exons and the lines represent introns (http://www.tigr.org/tdb/e2k1/ath1/). The mutant site and T-DNA insertion sites are shown in red. (C) Multi-sequence alignment analysis of Arabidopsis Shaker K+ channels. The mutant site (G315D) of Atlkt1 was located at the S6 region, which was conserved in Arabidopsis Shaker K+ channels. (D) RT-PCR verification of AtKC1 expression in Atkc1-1 (SALK_116451) and Atkc1-2 (SALK_140579) mutants. Elongation factor 1α (EF) was used as loading control. (E) Real-time PCR verification of AtKC1 expressions in two independent AtKC1-overexpressing lines (Col+AtKC1-1 and Col+AtKC1-2). (F) Phenotype comparisons of wild-type Arabidopsis (Col), Atkc1 mutants (Atlkt1, Atkc1-1 and Atkc1-2) and AtKC1-overexpressing lines (Col+AtKC1-1 and Col+AtKC1-2) after they are grown on MS and LK mediums for 4 days.
Map-based cloning was carried out using the F2 population, and the AtLKT1 was located within BAC clone, F4D11, of chromosome IV and finally identified to encode a Shaker K+ channel family α-subunit AtKC1 (At4g32650; Figure 1B). A single nucleotide mutation (G1473A) was found in Atlkt1 and it causes a change of Gly315 to Asp (G315D) in the amino acid sequence (Figure 1B and 1C). Protein sequence analysis shows that this mutation occurred in the S6 region, a highly conserved transmembrane domain in the Arabidopsis Shaker family (Figure 1C), which constitutes the pore structure of Shaker K+ channel together with P (pore) region and S5 region 22, 23, 24. Two T-DNA insertion mutant lines of AtKC1 gene (SALK_116451 and SALK_140579), ordered from ABRC, were used to test the LK-tolerant phenotype of Atlkt1 mutants in this study and they were named as Atkc1-1 and Atkc1-2, respectively (Figure 1B). The two T-DNA insertion sites of Atkc1-1 and Atkc1-2 locate in the second intron and the ninth exon of AtKC1, respectively, and both disrupt the transcription of AtKC1 gene (Figure 1B and 1D). Both T-DNA insertion mutants Atkc1-1 and Atkc1-2 showed the same LK-tolerant phenotype as the Atlkt1-mutant plants (Figure 1F; Supplementary information, Figures S1 and S2), suggesting that the LK-tolerant phenotype of these three allelic mutants all resulted from the loss function of the AtKC1 gene.
Two AtKC1-overexpressing lines (Col+AtKC1-1 and Col+AtKC1-2) were constructed by transforming the SUPER::AtKC1 vector into wild-type plants. The elevated expression levels of AtKC1 mRNA in roots of these two independent transgenic lines were verified by real-time PCR (Figure 1E). Phenotype tests show that both AtKC1-overexpressing lines displayed phenotypes similar to wild-type plants and their roots stopped growing on LK medium (Figure 1F; Supplementary information, Figures S1 and S2).
AtKC1 regulates K+ uptake and accumulation in Arabidopsis roots under LK conditions
The phenotype test results indicated that all the three Atkc1 mutants displayed significant tolerance to LK stress. It was further hypothesized that this LK-tolerant phenotype of root growth may be associated with the K+ uptake by roots. By determining K+ contents in various plant materials, we observed that the root K+ contents of two Atkc1 mutant lines (Atlkt1 and Atkc1-1) were significantly higher than that of wild-type plants and the AtKC1-overexpressing line (Col+AtKC1-1) after being cultured on LK medium for 4, 7 or 10 days (Figure 2A). The root K+ content of the AtKC1-overexpressing line was slightly lower than that of wild-type plants on LK medium (Figure 2A). However, the shoot K+ contents of these plants were not significantly different from each other under the same conditions (Figure 2B). In addition, there was no observed difference in both root and shoot K+ contents for these plants under MS conditions (Figure 2C and 2D). These results suggest that AtKC1 may play an important role in root K+ uptake in Arabidopsis, especially under LK conditions. The K+ content measurement results may also explain why the roots of the Atkc1 mutants can keep growing after being transferred to LK medium.
Comparison of K+ contents in various plant materials. (A and B) K+ contents of Arabidopsis roots (A) and shoots (B) from wild-type (Col), Atkc1 mutants (Atlkt1 and Atkc1-1) and AtKC1-overexpressing plants (Col+AtKC1-1) were measured after the seedlings were grown under the low-K+ (LK) conditions for the indicated time periods. Data are shown as means ± SE (n = 3). (C and D) K+ contents of roots (C) and shoots (D) from wild-type (Col), Atkc1 mutants (Atlkt1 and Atkc1-1) and AtKC1-overexpressing plants (Col+AtKC1-1) were measured after the seedlings were grown on the MS medium for the indicated time periods. Data are shown as means ± SE (n = 3). The Student's t-test (*P < 0.05) was used to analyze the statistical significance.
Furthermore, the ratios of root-to-shoot biomass for all tested plants were determined. Under the control conditions (on MS medium), all the tested plants showed similar root/shoot biomass ratios (Figure 3). The LK treatment significantly reduced the root/shoot biomass ratios of wild-type and AtKC1-overexpressing plants. However, the root/shoot ratios of Atkc1 mutants (Atlkt1 and Atkc1-1) were significantly increased under the LK conditions (Figure 3). These results suggest that AtKC1 may be involved in the regulation of biomass allocation between root and shoot under LK stress.
Comparison of root/shoot biomass ratios for various plant materials. The root/shoot biomass ratios of wild-type (Col), Atkc1 mutants (Atlkt1 and Atkc1-1) and AtKC1-overexpressing plants (Col+AtKC1-1) were measured after they are grown under the control conditions (MS) and low-K+ conditions (LK) for 10 days. Data are shown as means ± SE (n = 3). Student's t-test (*P < 0.05) was used to analyze the statistical significance.
AtKC1 regulates AKT1 activity
AtKC1 has been reported as an α-subunit of the Shaker K+ channel family primarily expressed in Arabidopsis roots 19, and AtKC1 alone does not seem to form a functional K+ channel in heterologous expression systems 17, 19. Our results showed that AtKC1 could not complement the growth phenotype of a yeast mutant strain with deficiency in K+ transport 25, indicating that AtKC1 may not directly function as a K+ transporter (data not shown). Considering that AtKC1 may be involved in K+ uptake regulation under LK stress (the results shown in Figures 1, 2, 3), together with the fact that the regulation of AKT1-mediated K+ uptake is related to Arabidopsis responses to LK stress 13, we hypothesized that AtKC1 may function as another regulator of AKT1 in addition to AtCIPK23. To test this hypothesis, first we detected the potential interaction between AKT1 and AtKC1. A previous study showed a strong physical interaction between the cytosolic regions of AKT1 and AtKC1 26. In present study, a similar interaction was detected in the yeast two-hybird assay (data not shown) despite using different AD and BD vectors (pACT2 and pGBT9 in an experiment carried out by Pilot et al. 26; pGADT7 and pGBKT7 in the present study).
Using the Xenopus oocyte heterologous expression system, our two-electrode voltage-clamp recording results showed that AKT1-mediated inward K+ currents were significantly inhibited by AtKC1 under hyperpolarization conditions when AKT1 cRNA and AtKC1 cRNA were coinjected at the ratio of 1:1 (Figure 4A) in the presence of AtCIPK23 and AtCBL1 13. However, substitution of AtKC1 with G315D (the mutant AtKC1 with a single amino acid change, G315D) did not inhibit AKT1-mediated inward K+ currents in oocytes (Supplementary information, Figure S3). The measured reversal potentials (Erev) of AKT1 currents (−57 ± 5 mV), AKT1/AtKC1 currents (−61 ± 3 mV) and AKT1/G315D currents (−58 ± 3 mV) all correlated well with the theoretical K+ equilibrium potentials (−60 mV) under the indicated conditions, suggesting that all observed currents were mediated by K+ ions.
AtKC1 inhibits AKT1-mediated inward K+ currents in Xenopus oocytes. (A) AKT1 current recordings in X. laevis oocytes. Whole-cell currents were recorded in oocytes with injection of different cRNAs: AKT1 (AKT1+AtCIPK23+AtCBL1) and AKT1+AtKC1 (AKT1+AtKC1+AtCIPK23+AtCBL1). The control oocytes were injected with water. The voltage protocols, as well as time and current scale bars for the recordings are shown. (B) I-V relationships of AKT1 steady-state currents in various oocytes. The ratios of injected cRNAs are indicated. The data are presented as means ± SE (n = 5). (C) The voltage dependence of inward K+ currents from various oocytes. The solid lines represented the best fits according to the Boltzmann functions: G/Gmax (rel. open probability)=1/(1+exp((Vm−V1/2)/S)). G (chord conductance) was calculated as G=I/(Vm−EK), where I is the steady-state current at voltage Vm, V1/2 is the membrane potential at which the chord conductance is half-maximal and S is a slope factor. The data are presented as means ± SE (n = 4). (D) The recording traces of oocyte membrane potentials (Vm) during the increment of extraoocyte K+ concentration. Current clamp technique was applied to record the changes of oocyte membrane potentials. Three arrows from left to right indicated the three shifts of the extraoocyte K+ concentration, respectively: 0.01 to 0.1 mM K+, 0.1 to 1 mM K+ and 1 to 10 mM K+. (E) Oocyte membrane potential changes (ΔVm) during the increment of extraoocyte K+ concentration.
In addition, we also tested whether the inhibition of AKT1 currents by AtKC1 in oocytes depended on the amount of injected AtKC1 cRNA or the ratio of AKT1 cRNA to AtKC1 cRNA (1:0.25 and 1:1). As shown in Figure 4B, along with the increment of injected AtKC1 cRNA, the inhibition of AKT1-mediated inward K+ currents was significantly enhanced. Meanwhile, the half-activation voltage (V1/2) of AKT1-mediated inward K+ currents was negatively shifted from −115 ± 1 mV (V1/2 AKT1) to −128 ± 2 mV (V1/2 AKT1:AtKC1=1:0.25) and to −165 ± 2 mV (V1/2 AKT1:AtKC1=1:1) (Figure 4C). Reversely, the AtKC1 mutant G315D shifted the voltage dependence of AKT1 channel more positively (V1/2 AKT1+G315D=−108 ± 2 mV) (Figure 4C). These results demonstrate that AtKC1 functions as a negative regulator of AKT1, and the mutant G315D reverses the inhibition of AtKC1 on AKT1 by altering the voltage dependence. The amino acid residue Gly315 in AtKC1 is highly conserved in S6 region of the Arabidopsis Shaker family (Figure 1C). A recent report showed that mutation of this amino acid residue in KAT1 (G286I) led to the shift of activation threshold to more negative voltage when compared with wild-type KAT1 27. Thus, the Gly residue in S6 region may be very important for the gating of K+ channels.
AtKC1 inhibits AKT1-mediated K+ uptake at low-K+ concentrations
On the basis of the results of phenotype analysis (Figure 1) and K+ content measurement (Figures 2 and 3), it seems that AKT1 regulation by AtKC1 mainly functions under LK conditions. Thus, the influence of AtKC1 on AKT1-mediated K+ uptake at low [K+]ext was also tested in the Xenopus oocyte heterologous expression system by analyzing the membrane potential changes of oocytes at low [K+]ext. The resting membrane potential of AKT1-expressing oocytes was hyperpolarized to −137 ± 7 mV when the K+ concentration in the bath solution decreased to 10 μM. Similar membrane hyperpolarization was also observed in the oocytes that were coinjected with AKT1 and G315D cRNAs (Table 1). However, the resting membrane potentials of control oocytes and AKT1/AtKC1 coinjected oocytes were −17 ± 6 mV and −18 ± 3 mV, respectively, when the K+ concentration in the bath solution decreased to 10 μM (Table 1). The membrane potential changes caused by [K+]ext increment were recorded (Figure 4D), and the steady-state membrane potentials at different [K+]ext were analyzed (Table 1). The recording results showed that the membrane potentials of AKT1-injected oocytes and AKT1/G315D coinjected oocytes were significantly depolarized when the [K+]ext was increased from 0.01 mM to 10 mM (Figure 4E and Table 1). However, the membrane potentials were not changed in the control oocytes and AKT1/AtKC1 coinjected oocytes when the [K+]ext was changed at a low concentration range (from 0.01 mM to 1 mM) (Figure 4E and Table 1). Considering the fact that the depolarization of membrane potential is related to K+ permeability of the membrane, these results demonstrate that AKT1 plays roles in K+ uptake at as low as 10 μM [K+]ext, and AtKC1 can inhibit AKT1-mediated K+ uptake at low [K+]ext.
AtKC1 shifts the voltage dependence of AKT1 channel in Arabidopsis root cells
Patch-clamp whole-cell recordings using Arabidopsis root cell protoplasts were also conducted in this study to further confirm the regulation of AKT1 channel by AtKC1 in vivo. The whole-cell recordings showed that there were 20% of Atkc1-mutant cells and AtKC1-overexpressing cells whose activation kinetics of inward K+ currents were different from wild-type cells. As shown in Figure 5A, the inward K+ currents of Atlkt1 mutants were more easily activated (negative than −60 mV) compared to wild-type (negative than −80 mV) and AtKC1-overexpressing plants (negative than −120 mV). Comparing with wild-type plants, the half-activation voltages (V1/2) of Atlkt1 mutant and the AtKC1-overexpressing line were positively and negatively shifted, respectively (Figure 5B). These findings are consistent with the observations in Xenopus oocytes that the half-activation voltage (V1/2) of AKT1/AtKC1 heteromeric channel was shifted to the more negative direction compared with AKT1 channel.
AtKC1 regulates AKT1-mediated K+ currents in Arabidopsis root cells. (A) Patch-clamp whole-cell recordings in Arabidopsis root cell protoplasts. The plant materials used for root cell protoplast isolation are indicated above each recording. The voltage protocols, as well as time and current scale bars for the recordings are shown inside the figure. (B) The voltage dependence of the inward K+ currents from the root cell protoplasts isolated from wild-type (Col), Atkc1 mutants (Atlkt1) and AtKC1-overexpressing plants (Col+AtKC1-1). The solid lines represented the best fits according to the Boltzmann functions: G/Gmax (rel. open probability)=1/(1+exp((Vm−V1/2)/S)).
Discussion
AtKC1, a 'silent' but 'regulatory' component for functional K+ channels
Many inward K+ channel α-subunits from the plant Shaker family have been successfully expressed and functionally characterized in various heterologous expression systems 8. Most of them show similar channel properties, such as hyperpolarization activation and high K+ selectivity. Nevertheless, AtKC1, a unique K+ channel α-subunit, has not been functionally characterized so far. Neither the inward currents nor the outward currents were recorded in the heterologous cells where AtKC1 was expressed alone 17, 19, unpublished observations in this lab]. The localization of AtKC1 on the endoplasmic reticulum but not on the plasma membrane was the possible reason leading to no current of AtKC1 in heterologous cells 20. On the other hand, yeast two-hybrid results showed that the AtKC1 subunit did not interact with itself 26, suggesting that AtKC1 may not form a homomeric K+ channel. Although AtKC1 alone is a 'silent' K+ channel 19, some evidence has shown that AtKC1 may act as a regulatory α-subunit to modulate the activity of other K+ channels 17, 19, 20, 21.
It is well known that many animal K+ channels could assemble together and form heteromeric K+ channels 14, 15, 16. This heteromerization was also observed in plants 18. Yeast two-hybrid analysis indicated that most inward-rectifying K+ channels within the Shaker family could physically interact with each other 6. The AtKC1 subunit can interact with AKT1 and KAT1 20, 26. Electrophysiological evidence further confirmed that AtKC1 could assemble with other K+ channel subunits to form functional heteromeric K+ channels with novel channel properties 19, 20, 21. Considering all these reported evidence together with the present results, one may conclude that AtKC1 is an important regulatory component for various K+ channels in plant cells, although AtKC1 itself is 'silent' in terms of a functional channel.
A recent study 20 has shown that AtKC1 regulates AKT1 channel activity when they were coexpressed in tobacco mesophyll protoplasts. The activation threshold voltage of AKT1/AtKC1 heteromeric channels was negatively shifted compared with that of AKT1 homomeric channels. Using another heterologous expression system, namely, Xenopus oocyte, in the present study, we observed similar regulation between AKT1 and AtKC1 (Figure 4). Our results of patch-clamp experiments using Arabidopsis root cell protoplasts further confirmed this regulation in vivo (Figure 5B). There were 20% of Atkc1-mutant cells and AtKC1-overexpressing cells whose activation kinetics of inward K+ currents were obviously changed. Moreover, AtKC1 could inhibit the channel activities of some other K+ channels as well, for example, KST1 and KAT1 17, 20. In addition, KDC1, the homolog of AtKC1 form Daucus carota, also showed the similar inhibition on KAT1 channel when they were coexpressed in Xenopus oocytes 28. All these evidence supports the notion that AtKC1 acts as an inhibitory α-subunit when forming heteromeric channels with other K+ channel α-subunits.
Two expression systems, Xenopus oocytes and tobacco mesophyll protoplasts, seem to be both suitable for the heterologous characterization of AKT1, because AKT1 exhibits similar gating properties in these two systems compared with those in native Arabidopsis root cells 13, 19, 20, 29. The tobacco mesophyll protoplasts seem to be a more realistic expression system than animal cells when studying the plant ion channel regulation 29. However, a drawback of this system was observed. The current amplitude and voltage threshold of AKT1 or AKT1/AtKC1 channel recorded from tobacco mesophyll protoplasts showed significant variation, probably resulting from the different expression levels of AKT1 and AtKC1 in different transformed protoplasts 20. In addition, the orthologues of AKT1 and AtKC1, named NKT1 and NtKC1, respectively, were identified in tobacco leaves and cultured cells 30, 31. The possibilities of endogenous NKT1 as well as NtKC1 affecting AKT1 channel in AKT1-transformed tobacco mesophyll protoplasts could not be excluded, despite the fact that the potassium currents recorded from AKT1-transformed protoplasts exhibited similar properties close to those observed in Atkc1-mutant plants 19, 29. Comparatively, the Xenopus oocyte system may be more suitable for the regulatory mechanism investigation of AtKC1 on AKT1 for the following reasons. First, the expression level and current amplitude of AKT1 or AKT1/AtKC1 channels in oocytes were stable and could be easily controlled by altering the cRNA injection amount, which leads to the precise analysis of channel properties. Second, the AKT1 potassium currents recorded in oocytes were very similar to those in Atkc1-mutant plants, suggesting few endogenous modulators affecting AKT1 channel activity in oocytes. Third, the possible influence by homologous plant K+ channels (for example, NKT1, NtKC1) was completely excluded in Xenopus oocytes. Despite the successful characterization of AKT1 in heterologous systems, the in vivo study in Arabidopsis could not be substituted. Thus, in the present study, the regulation of AKT1 by AtKC1 was first investigated in Xenopus oocytes, and then was confirmed in Arabidopsis root cell protoplasts isolated from different plant materials. The results derived from both in vitro and in vivo analyses were highly consistent (Figures 4C and 5B), which clearly demonstrated the regulation of AKT1 by AtKC1.
Physiological importance of AKT1 inhibition by AtKC1
Plants may develop an adaptive mechanism by which they could survive under LK stress. As a voltage-dependent K+ channel, AKT1 can sense the changes of membrane potentials and can be activated (opened) at hyperpolarized potentials, such as those more negative than −80 mV. However, activation of AKT1 may result in net K+ efflux under LK conditions. For example, when the [K+]ext is decreased to 100 μM, the K+ equilibrium potential (EK) is −180 mV (assuming the cytosolic K+ concentration is about 100 mM). In this case, if the actual membrane potential is negative to −80 mV but does not reach −180 mV, AKT1 may open and cytosolic K+ efflux driven by the transmembrane K+ gradient may occur. It has been reported that AKT1 homomeric channels could result in K+ leakage at low [K+]ext when expressed in tobacco mesophyll protoplasts 20 or in Xenopus oocytes 21. Nevertheless, due to the presence of AtKC1, the activation voltage threshold of AKT1 channels is negatively shifted so that AKT1 may not be activated. As a result, possible K+ efflux through AKT1 channels is inhibited and K+ loss is limited 18, 21, 32.
Deficiency of essential mineral elements significantly inhibits the plant growth and development, and results in the change of plant root-to-shoot (R:S) biomass ratio 33, 34. The plants accumulate sugars in leaves when they suffer K+ deficiency, but the sugar transport from shoot to root via phloem is impaired in this circumstance. As a result, the root growth is limited because of the lack of sugars, and their R:S biomass ratio is decreased 35, 36, 37. Our results in this study showed that the R:S biomass ratio in wild-type plants was reduced when the seedlings were subjected to the LK stress (Figure 3). Contrarily, the Atkc1 mutants significantly increased their R:S biomass ratios under LK conditions (Figure 3). These results clearly demonstrate that AtKC1 not only functions in regulation of root K+ uptake but also plays a role in biomass allocation between root and shoot under the LK conditions. It is further hypothesized that the increment of K+ uptake in the Atkc1-mutant roots may resume the sugar transport from shoot to root, which results in the accumulation of sufficient carbon sources in the roots of Atkc1 mutants so that the mutants can maintain their root growth under LK conditions.
A complex K+ uptake regulatory pathway in Arabidopsis in response to LK stress
Plants may have a complex system for their K+ acquisition, and all the involved K+ transporters in this system must be precisely controlled and regulated. Previous studies have shown that posttranslational regulation is an important mechanism for regulation of K+ channel activities in plant cells 13, 38, 39, 40. One of the examples is the regulation of AKT1 activity by a protein kinase AtCIPK23. After activation by the calcium sensor AtCBL1 or AtCBL9, AtCIPK23 directly phosphorylates AKT1 and activates the AKT1-mediated K+ influx 13. In this positive regulatory pathway, AKT1 is activated by a phosphorylation process. In the present study, we show a negative regulatory pathway of AKT1. AtKC1 functions as another modulator of AKT1 and negatively regulates the AKT1 activity. These two regulatory pathways seem both important for plant responses to low-K+ stress and may both participate in the equilibrium of K+ uptake and K+ homeostasis in plants, especially under low-K+ stress conditions. Obviously, there remain a number of unknown events in plant signaling mechanisms in response to LK stress. For example, what are the initial signaling mechanisms in plant response to LK stress? How are the different regulatory mechanisms for AKT1 coordinated? Is there any other component(s) for AKT1 regulation?
In addition, transcriptional regulation of K+ transporters is another important mechanism for plants in response to environmental stresses. It was reported that K+ deprivation may induce the transcriptional expression of some K+ transporters 41, 42, 43. However, K+ deficiency did not alter AKT1 transcription 26, 44, 45. Our observations showed that overexpressing AKT1 in the 35S::AKT1 Arabidopsis line did not change their phenotype under LK stress (unpublished data). These results suggest that the regulation of AKT1 may occur at posttranslational level. Unlike AKT1, transcription of AtKC1 was significantly influenced when the Arabidopsis plants were subjected to various environmental stimuli, such as K+ starvation, salt stress and hormone treatment 26, 46. Taking all these evidence into account, an AtKC1-mediated regulatory pathway for K+ uptake in Arabidopsis plants under LK stress is proposed as shown in Figure 6. Plants may sense LK stress and subsequently increase the transcriptional level of AtKC1. The AtKC1 protein, as a regulatory subunit, could assemble with other Shaker K+ channel subunits (such as AKT1) and form heteromeric K+ channels with novel channel properties. Consequently, the plant K+ uptake and translocation are accurately regulated, which may help plants to adapt to the environmental changes.
The Shaker family members AKT1 and AtKC1, as two important K+ channels mainly expressed in Arabidopsis roots, play crucial roles in K+ acquisition from the environment. AKT1 has been confirmed as a functional K+ channel conducting the K+ influx in Arabidopsis root cells. Here, we show the evidence for the regulatory function of AtKC1 in the AKT1-mediated root K+ uptake, especially under LK conditions. This study demonstrates that the 'silent' K+ channel α-subunit AtKC1 negatively regulates the AKT1-mediated K+ uptake in Arabidopsis roots and consequently alters the R:S ratio under the LK stress conditions. AtKC1 subunit may assemble with AKT1 forming heteromeric K+ channels and inhibit the channel activities of AKT1 in terms of activation voltage. K+ channel heteromerization has been considered as an important mechanism to regulate K+ uptake and transport in plants, which may confer plants more 'adaptive' or 'survival' abilities in response to environmental stresses.
Materials and Methods
Mutant isolation, plant materials and growth conditions
For the A. thaliana LK-tolerant (Atlkt) mutant isolation, EMS-mutagenized M2 seeds of Arabidopsis (Columbia ecotype) were germinated on MS medium containing 0.8% (w/v) agar and 3% (w/v) sucrose at 22 °C under constant illumination at 60 μmol m−2 s−1 for 4 days. Then, M2 seedlings were transferred to the LK (100 μM K+) medium to screen for the putative Atlkt mutants, whose main root could still keep growing on LK medium. The LK medium was prepared by modification of MS medium described previously 13. The putative Atlkt mutants were grown under the normal conditions to harvest M3 seeds and the Atlkt-mutant phenotype was further confirmed using M3 seedlings on the LK medium. The selected M3 mutants were backcrossed with the wild-type plants and phenotypes of their F1 and F2 generations were tested on the LK medium for genetic analysis. For seed harvest and hybridization, Arabidopsis plants were grown in potting soil mixture (rich soil:vermiculite = 2:1, v/v) and kept in growth chambers at 22 °C with illumination at 120 μmol m−2 s−1 for a 16 h daily light period. The relative humidity was ∼70% (± 5%).
Map-based cloning of AtLKT1
The Atlkt1 (Columbia background) mutants were crossed with Landsberg erecta wild-type plants to create mapping population. Totally, 2 217 individual F2 plants were selected for chromosomal mapping of AtLKT1.
Vector constructions and Arabidopsis transformation
The SUPER::AtKC1 construct was generated by cloning the coding sequence of AtKC1 into pBIB vector under control of the SUPER promoter 47. Arabidopsis transformation with Agrobacterium (strain GV3101) was carried out by the floral dip method 48.
Biomass and potassium content measurement
Arabidopsis seedlings (4-day-old) were transferred from MS medium to the LK medium and treated for different time periods as indicated. The root and shoot tissues were harvested separately and placed in an oven drying at 80 °C for 48 h. Then, the dry weights of samples were measured as dry biomass. The root/shoot ratios of dry weights were calculated. For the K+ content measurement, the dry plant tissues were treated in a muffle furnace at 575 °C for 5 h and then dissolved in 0.1 M HCl. Potassium concentrations of the samples were measured using atomic absorption spectrophotometry (Hitachi Z-5000).
In vitro transcription and expression in Xenopus Oocytes
The coding sequence of AKT1, AtKC1, G315D, AtCIPK23 and AtCBL1 were cloned into pGEMHE vector. The cRNAs were transcribed in vitro using the T7 RiboMAX Large-Scale RNA Production System (Promega). The oocytes were isolated from X. laevis and injected with cRNAs. The oocytes injected with 50 nl distilled water were taken as control. The AKT1 expressing oocytes were injected with the cRNA mixture of AKT1, AtCIPK23 and AtCBL1 (6:6:6 ng in 50 nl). The AKT1 and AtKC1 (or G315D) coexpressed oocytes were injected with cRNA mixture of AKT1, AtKC1 (or G315D), AtCIPK23 and AtCBL1 (6:6:6:6 ng in 50 nl). To analyze the AtKC1 dependence of AKT1 currents, the injected AtKC1 cRNA was reduced from 6 ng to 1.5 ng, and other cRNAs were not changed. Before being used for voltage-clamp recordings, the injected oocytes were incubated at 17 °C in the modified Barth's solution containing (in mM) 88 NaCl, 1.0 KCl, 0.91 CaCl2, 0.33 Ca(NO3)2, 0.82 MgSO4, 2.4 NaHCO3 and 10 HEPES-NaOH (pH 7.5), supplemented with gentamycin (0.1 mg ml−1) and streptomycin (0.1 mg ml−1).
Two-electrode voltage-clamp recording from Xenopus oocytes
Whole-cell recordings were performed 2 days after cRNA injection. A two-electrode voltage-clamp technique was applied using a GeneClamp 500B amplifier (Axon Instruments Inc) at room temperature (∼20 °C). The microelectrodes were filled with 3 M KCl. The bath solution contains (in mM) 10 KCl, 1.8 MgCl2, 1.8 CaCl2, 180 D-mannitol and 10 MES-Tris (pH 5.8). The whole-cell currents were filtered at 1 kHz and digitized through a Digidata 1322A AC/DC converter using Clampex9.0 software (Axon Instruments Inc).
Patch-clamp whole-cell recording from root cell protoplasts
Root cell protoplasts were isolated from 5-day-old primary roots of Arabidopsis seedlings 13. Standard whole-cell recording techniques were applied. The contents of the bath and pipette solutions for the whole-cell recordings are the same as described 12, 13. The patch-clamp recordings were conducted at room temperature (20 °C ± 2 °C) in dim light. Whole-cell currents were recorded using an Axopatch-200A amplifier (Axon Instruments Inc) connected to a computer via an interface (TL-1 DMA Interface, Axon Instruments).
References
Clarkson DT, Hanson JB . The mineral nutrition of higher plants. Annu Rev Plant Physiol 1980; 31:239–298.
Véry AA, Sentenac H . Molecular mechanisms and regulation of K+ transport in higher plants. Annu Rev Plant Biol 2003; 54:575–603.
Schroeder JI, Ward JM, Gassmann W . Perspectives on the physiology and structure of inward-rectifying K+ channels in higher plants: biophysical implications for K+ uptake. Annu Rev Biophys Biomol Struct 1994; 23:441–471.
Pettersson S, Jensen P . Variation among species and varieties in uptake and utilization of potassium. Plant Soil 1983; 72:231–237.
Mengel K, Kirkby EA . Potassium. In: Principles Of Plant Nutrition. Noewell: Kluwer Academic Publishers, 2001: 503–509.
Lebaudy A, Véry AA, Sentenac H . K+ channel activity in plants: genes, regulations and functions. FEBS Lett 2007; 581:2357–2366.
Gierth M, Mäser P . Potassium transporters in plants - involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett 2007; 581:2348–2356.
Gambale F, Uozumi N . Properties of Shaker-type potassium channels in higher plants. J Membr Biol 2006; 210:1–19.
Lagarde D, Basset M, Lepetit M, et al. Tissue-specific expression of Arabidopsis AKT1 gene is consistent with a role in K+ nutrition. Plant J 1996; 9:195–203.
Hirsch RE, Lewis BD, Spalding EP, Sussmanm MR . A role for the AKT1 potassium channel in plant nutrition. Science 1998; 280:918–921.
Spalding EP, Hirsch RE, Lewis DR, Qi Z, Sussman MR, Lewis BD . Potassium uptake supporting plant growth in the absence of AKT1 channel activity: inhibition by ammonium and stimulation by sodium. J Gen Physiol 1999; 113:909–918.
Ivashikina N, Becker D, Ache P, Meyerhoff O, Felle HH, Hedrich R . K+ channel profile and electrical properties of Arabidopsis root hairs. FEBS Lett 2001; 508:463–469.
Xu J, Li HD, Chen LQ, et al. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 2006; 125:1347–1360.
Salinas M, Duprat F, Heurteaux C, Hugnot JP, Lazdunski M . New modulatory alpha subunits for mammalian Shab K+ channels. J Biol Chem 1997; 272:24371–24379.
Stocker M, Hellwig M, Kerschensteiner D . Subunit assembly and domain analysis of electrically silent K+ channel alpha-subunits of the rat Kv9 subfamily. J Neurochem 1999; 72:1725–1734.
Ottschytsch N, Raes A, Van Hoorick D, Snyders DJ . Obligatory heterotetramerization of three previously uncharacterized Kv channel alpha-subunits identified in the human genome. Proc Natl Acad Sci USA 2002; 99:7986–7991.
Dreyer I, Antunes S, Hoshi T, et al. Plant K+ channel α-Subunits assemble indiscriminately. Biophys J 1997; 72:2143–2150.
Lebaudy A, Hosy E, Simonneau T, et al. Heteromeric K+ channels in plants. Plant J 2008; 54:1076–1082.
Reintanz B, Szyroki A, Ivashikina N, et al. AtKC1, a silent Arabidopsis potassium channel α-subunit modulates root hair K+ influx. Proc Natl Acad Sci USA 2002; 99:4079–4084.
Duby G, Hosy E, Fizames C, et al. AtKC1, a conditionally targeted Shaker-type subunit, regulates the activity of plant K+channels. Plant J 2008; 53:115–123.
Geiger D, Becker D, Vosloh D, et al. Heteromeric AtKC1/AKT1 channels in Arabidopsis roots facilitate growth under K+-limiting conditions. J Biol Chem 2009; 284:21288–21295.
Durell SR, Guy HR . Structural models of the KtrB, TrkH, and Trk1, 2 symporters based on the structure of the KcsA K+ channel. Biophys J 1999; 77:789–807.
Durell SR, Hao Y, Nakamura T, Bakker EP, Guy HR . Evolutionary relationship between K+ channels and symporters. Biophys J 1999; 77:775–788.
Kato Y, Sakaguchi M, Mori Y, et al. Evidence in support of a four transmembrane-pore-transmembrane topology model for the Arabidopsis thaliana Na+/K+ translocating AtHKT1 protein, a member of the superfamily of K+ transporters. Proc Natl Acad Sci USA 2001; 98:6488–6493.
Ko CH, Gaber RF . TRK1 and TRK2 encode structurally related K+ transporters in Saccharomyces cerevisiae. Mol Cel Biol 1991; 11:4266–4273.
Pilot G, Gaymard F, Mouline K, Chérel I, Sentenac H . Regulated expression of Arabidopsis Shaker K+ channel genes involved in K+ uptake and distribution in the plant. Plant Mol Biol 2003; 51:773–787.
Gajdanowicz P, Garcia-Mata C, Gonzalez W, et al. Distinct roles of the last transmembrane domain in controlling Arabidopsis K+ channel activity. New Phytol 2009; 182:380–391.
Naso A, Montisci R, Gambale F, Picco C . Stoichiometry studies reveal functional properties of KDC1 in plant shaker potassium channels. Biophys J 2006; 91:3673–3683.
Hosy E, Duby G, Véry AA, Costa A, Sentenac H, Thibaud JB . A procedure for localisation and electrophysiological characterisation of ion channels heterologously expressed in a plant context. Plant Methods 2005; 1:1–14.
Sano T, Becker D, Ivashikina N, et al. Plant cells must pass a K+ threshold to re-enter the cell cycle. Plant J 2007; 50:401–413.
Dai XY, Su YR, Wei WX, Wu JS, Fan YK . Effects of top excision on the potassium accumulation and expression of potassium channel genes in tobacco. J Exp Bot 2009; 60:279–289.
Shabala S, Cuin TA . Potassium transport and plant salt tolerance. Physiol Plant 2008; 133:651–669.
Marschner H . Mineral Nutrition of Higher Plants. 2nd Edition. London: Academic Press, 1995.
Hermans C, Hammond JP, White PJ, Verbruggen N . How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci 2006; 11:610–617.
Cakmak I, Hengeler C, Marschner H . Partitioning of shoot and root dry matter and carbohydrates in bean plants suffering from phosphorus, potassium and magnesium deficiency. J Exp Bot 1994; 45:1245–1250.
Cakmak I, Hengeler C, Marschner H . Changes in phloem export of sucrose in leaves in response to phosphorus, potassium and magnesium deficiency in bean plants. J Exp Bot 1994; 45:1251–1257.
White PJ . The regulation of K+ influx into roots of rye (Secale cereale L.) seedlings by negative feedback via the K+ flux from shoot to root in the phloem. J Exp Bot 1997; 48:2063–2073.
Zhang X, Ma J, Berkowitz GA . Evaluation of functional interaction between K+ channel α- and β-subunits and putative inactivation gating by co-expression in Xenopus laevis oocytes. Plant Physiol 1999; 121:995–1002.
Sottocornola B, Visconti S, Orsi S, et al. The potassium channel KAT1 is activated by plant and animal 14-3-3 proteins. J Biol Chem 2006; 281:35735–35741.
Chérel I, Michard E, Platet N, et al. Physical and functional interaction of the Arabidopsis K+ channel AKT2 and phosphatase AtPP2CA. Plant Cell 2002; 14:1133–1146.
Kim EJ, Kwak JM, Uozumi N, Schroeder JI . AtKUP1: an Arabidopsis gene encoding high-affinity potassium transport activity. Plant Cell 1998; 10:51–62.
Ahn SJ, Shin R, Schachtman DP . Expression of KT/KUP genes in Arabidopsis and the role of root hairs in K+ uptake. Plant Physiol 2004; 134:1135–1145.
Gierth M, Mäser P, Schroeder JI . The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol 2005; 137:1105–1114.
Maathuis FJ, Filatov V, Herzyk P, et al. Transcriptome analysis of root transporters reveals participation of multiple gene families in the response to cation stress. Plant J 2003; 35:675–692.
Hampton CR, Bowen HC, Broadley MR, et al. Cesium toxicity in Arabidopsis. Plant Physiol 2004; 136:3824–3837.
Shin R, Schachtman DP . Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc Natl Acad Sci USA 2004; 101:8827–8832.
Li X, Gong Z, Koiwa H, et al. Bar-expressing peppermint (Mentha × Piperita L. var. Black Mitcham) plants are highly resistant to the glufosinate herbicide Liberty. Mol Breed 2001; 8:109–118.
Clough SJ, Bent AF . Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 1998; 16:735–743.
Acknowledgements
We thank Dr Emily Liman (University of Southern California, USA) for providing the pGEMHE vector for the Xenopus oocyte experiments. We also thank Dr Richer Gaber (Northwestern University, USA) for providing the yeast mutant strain with K+ transport deficiency. We are grateful to Dr Rainer Hedrich (University of Würzburg, Germany) for critical discussion. This work was supported by the National Natural Science Foundation of China (grant no. 30830013 to WHW), the Beijing Municipal Education Commission (grant no. YB20081001901 to WHW) and the Program of Introducing Talents of Discipline to Universities (grant no. B06003 to WHW).
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Phenotype comparisons of various plant materials under normal photoperiod conditions. (PDF 1370 kb)
Supplementary information, Figure S2
Phenotype comparisons of various plant materials germinating under LK conditions. (PDF 684 kb)
Supplementary information, Figure S3
AKT1-mediated inward K+ currents in Xenopus oocytes when co-expressed with G315D (PDF 210 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., He, L., Li, HD. et al. Potassium channel α-subunit AtKC1 negatively regulates AKT1-mediated K+ uptake in Arabidopsis roots under low-K+ stress. Cell Res 20, 826–837 (2010). https://doi.org/10.1038/cr.2010.74
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2010.74
Keywords
This article is cited by
-
Comparative physiological and transcriptome analysis between potassium-deficiency tolerant and sensitive sweetpotato genotypes in response to potassium-deficiency stress
BMC Genomics (2024)
-
Impact of potassium starvation on the uptake, transportation, photosynthesis, and abiotic stress tolerance
Plant Growth Regulation (2023)
-
Dealing with Environmental Fluctuations: Diversity of Potassium Uptake Systems Across the Three Domains of Life
Journal of Plant Growth Regulation (2023)
-
Major-effect quantitative trait locus qLKR4.1 encodes a phospholipase Dδ protein associated with low-K+ stress tolerance by promoting root length
Theoretical and Applied Genetics (2023)
-
Whole-genome resequencing of wild and cultivated cannabis reveals the genetic structure and adaptive selection of important traits
BMC Plant Biology (2022)