Abstract
A potential link between inflammation and cancer has been suspected for over a century, but the exact molecular mechanisms connecting the two remained nebulous. We proposed that NF-κB transcription factors regulated via the IκB kinase (IKK) complex play a critical role in coupling inflammation and cancer and have set out to test this hypothesis in mouse models of cancer. Using mice bearing mutations in the genes coding for the IKKβ and IKKα catalytic subunits we obtained evidence supporting a critical role for IKKβ in tumor promotion and more recently identified the involvement of IKKα in metastatogenesis. Whereas the major pro-tumorigenic function of IKKβ is mediated via NF-κB, the pro-metastatic function of IKKα is NF-κB-independent. In addition to illustrating the critical roles of the two IKK molecules in linking inflammation and cancer and providing an explanation for increased cancer risk in response to persistent infections and inflammation, these results also identify new targets for development of novel anti-cancer therapies and preventive strategies. Instead of targeting the cancer cell itself, as done by conventional anti-cancer drugs, the new therapeutics will target processes that occur within inflammatory cells that are essential for cancer development and progression. Unlike cancer cells, inflammatory cells retain a normal and stable genome and therefore are unlikely to become genetically resistant to therapeutic intervention.
Similar content being viewed by others
Introduction
An association between inflammation and cancer was noted by Virchow in the 19th century 1 and has been supported by more recent epidemiological data that led to the estimate that approximately 20% of cancer deaths are linked to chronic infections and persistent inflammation 2. Primary examples are gastric cancer and Helicobacter pylori infections 3, hepatocellular carcinoma (HCC) and viral hepatitis 4 and colitis-associated cancer (CAC) 5. Yet, epidemiological associations do not establish causality and the mechanisms that bridge inflammation and cancer were only recently studied. Initial work had demonstrated the importance of tumor necrosis factor (TNF)-α and its type I TNF-α receptor (TNFR1) in the development of squamous cell carcinoma (SCC) induced by two-stage chemical carcinogenesis 6. The molecular mechanism by which TNFR1 signaling promotes SCC development, however, has not been fully explained although it is thought to be mediated via protein kinase C (PKC)α and activator protein 1 (AP-1) transcriptional factors 7. Another cytokine, colony stimulating factor-1 (CSF-1), is required for progression of fully malignant mammary carcinoma in mice, presumably through its effect on macrophage development and function 8. The mechanisms by which macrophages stimulate the development and progression of mammary carcinoma are still being elucidated 9. Another mediator of inflammation, the enzyme cyclo-oxygenase 2 (COX2), responsible for inducible synthesis of prostanoids 10, is required for development of colonic polyps and adenomas in Apc+/min mice 11. Accordingly, COX2 inhibitors, including non-steroidal anti-inflammatory drugs (NSAIDs), were found in large-scale clinical trials to prevent progression of adenomatous polyposis coli (APC) to colorectal adenocarcinomas, as well as reduce the overall incidence of colorectal cancer 7, 12, 13. Contribution of COX2 to the progression of colorectal cancer may be mediated via PGE2, which stimulates angiogenesis and other processes 12.
In an attempt to explain the molecular underpinnings that link inflammation and cancer, we proposed that transcription factor NF-κB, formed through combinatorial dimerization of 5 family members 14, is the key molecular lynchpin that connects the two 15. This hypothesis was based on a large body of circumstantial evidence, such as the frequent presence of constitutively activated NF-κB in diverse solid malignancies 15 and the well established ability of NF-κB to upregulate production of key pro-inflammatory cytokines and enzymes, including TNF-α, IL-1, IL-6, CSF-1 and COX-2 16, as well as induce the expression of genes that code for anti-apoptotic proteins 17, 18. We proposed that persistent infections and chronic inflammation result in NF-κB activation and once induced, NF-κB target genes protect pre-neoplastic and fully malignant cells from apoptosis induced by surveillance mechanisms that are activated by DNA damage and chromosomal rearrangements or by genotoxic anti-cancer drugs and radiation. NF-κB activation, we suggested, contributes not only to the emergence and expansion of pre-neoplastic cells but can also confer drug and radiation resistance upon fully developed tumors. Indeed, a role for NF-κB in drug and radiation resistance was demonstrated by Baldwin and colleagues 19. In this context it is worth mentioning that we recently found that in addition to inhibition of apoptosis, NF-κB can also prevent necrosis 20, a form of cell death that may be more relevant to the mode of action of many anti-cancer drugs 21.
The mechanisms and pathways responsible for NF-κB activation in solid malignancies (mainly carcinomas) are still not fully elucidated and in most cases are unlikely to be due to direct mutational activation 15. During chronic infections NF-κB becomes activated in response to production of pathogen associated molecular patterns (PAMPs) or through pro-inflammatory cytokines such as TNF-α and IL-1, during persistent inflammation. These and other mechanisms may also activate NF-κB in cancer cells. Most NF-κB activators act via diverse cell surface receptors that impinge on a common molecular target – the IκB kinase (IKK) complex 22, 23. The IKK complex consists of two catalytic subunits: IKKα and IKKβ, and a regulatory subunit IKKγ/NEMO. Gene disruption via homologous recombination revealed that activation of NF-κB in response to PAMPs and pro-inflammatory cytokines is dependent on IKKγ 24 and quite often on IKKβ 25, 26, 27. By contrast, IKKα, but not IKKβ, kinase activity is required for activation of an alternative NF-κB signaling pathway based on processing of NF-κB2/p100:RelB complexes to NF-κB2/p52:RelB dimers 28. In addition, IKKα, and not IKKβ, is required for differentiation of stratified epithelia, such as the epidermis, but this function does not require its protein kinase activity 27, 29, 30. Given the critical role of IKKβ in activation of the classical NF-κB signaling pathway in response to PAMPs and pro-inflammatory cytokines, we used targeted Ikkβ gene disruptions to study the role of IKKβ-dependent NF-κB activation in a variety of cancer models in mice.
Epithelial and myeloid cell NF-κB are important for colitis-associated cancer
We first examined the cancer promoting role of IKKβ in a mouse model of CAC, that relies upon azoxymethane (AOM), a procarcinogen that is metabolically activated in colonic epithelial cells and dextran sulfate sodium salt (DSS), an irritant that induces colonic inflammation 31. Administration of either AOM or DSS alone is not sufficient for effective tumor induction but both agents together cause efficient formation of adenomas and adenocarcinomas with 100% penetrance in C57BL6 mice. Like human CAC, these tumors appear at the distal part of the colon and show activation of the Wnt/β catenin pathway. Using mice homozygous for a “floxed” IkkβF allele 26 that have been genetically crossed to Villin-Cre mice that express Cre recombinase in intestinal epithelial cells (IEC), we examined the contribution of IKKβ in IEC to CAC development. Prior to that we found that unchallenged IkkβF/F/Villin-Cre mice (IkkβΔIEC) are healthy and exhibit normal colon structure and function 26. Importantly, IkkβΔIEC mice exhibited a striking 80% decline in CAC load relative to similarly treated wild type (WT) mice, thereby providing the first conclusive evidence for the role of IKKβ-dependent NF-κB activation in development of an inflammation-promoted cancer 32. IkkβΔIEC mice lack IKKβ in the very same cells that are subject to the mutagenic activity of AOM and give rise to the genetically transformed component of CAC – the malignant adenocarcinoma cell, which frequently contains β-catenin (Catnb) mutations. However, another important cell type in inflammation-promoted colon cancer is the lamina propria macrophage, a critical source of inflammatory cytokines and mediators 33, 34. To investigate the role of IKKβ in these cells during CAC development, we introduced into the IkkβF/F strain a Cre transgene driven by the LysM promoter, which is active in mature macrophages and neutrophils 35. IkkβF/F/LysM-Cre mice (IkkβΔmye), which are also healthy and physiologically normal when kept unchallenged, exhibited a 50% decrease in tumor multiplicity, but a greater decline in total tumor load, as most of the tumors presented by these mice were smaller in size relative to tumors in WT mice 32. These results provided the first genetically supported evidence for the tumor promoting role of NF-κB activation in inflammatory cells, in this case the lamina propria macrophage or a dendritic cell.
Although both IkkβΔIEC and IkkβΔmye mice display reduced tumor load, detailed analysis revealed that in each cell type, IKKβ-driven NF-κB promotes tumor development through a different mechanism. In the IEC, the most important tumor promoting function of NF-κB is to endow the emerging pre-malignant cell with a survival advantage by inducing anti-apoptotic genes, such as Bcl-XL, whose products prevent culling of transformed cells through genomic surveillance mechanisms. It should be noted, however, that the IKKβ deficiency in the IEC had no effect on cell proliferation or the spectrum of oncogenic mutations induced by AOM, most of which affect the Catnb gene 32. In myeloid cells, on the other hand, IKKβ-driven NF-κB promotes tumor development through induction of growth factors that stimulate proliferation and expansion of pre-neoplastic cells 32. One of these factors was suggested to be IL-6 36. We recently confirmed the important role of IL-6, which is encoded by a typical NF-κB target gene, in CAC development using Il6−/− mice and reaffirmed our earlier findings that during the initial stages of the CAC protocol the main producer of IL-6 is the myeloid cell (E Karin, J Terzic and S Grivennikov, unpublished results).
These studies have provided critical support for the important tumor promoting function of NF-κB. These studies have also demonstrated for the first time, that IKKβ-driven NF-κB mostly affects tumor promotion rather than tumor initiation and that it can act through distinct mechanisms in different cell types. In this case, NF-κB in IEC prevents apoptotic elimination of pre-neoplastic cells, whereas NF-κB in lamina propria inflammatory cells induces the production of growth factors, such as IL-6, that stimulate the proliferation of these cells (Figure 1).
IKKβ-driven NF-κB promotes the development of colitis-associated cancer (CAC) by acting in two different celltypes. NF-κB activation in pre-malignant intestinal epithelial cells (IEC carrying oncogenic mutations induced by AOM exposure) results in upregulation of pro-survival genes such as Bcl-XL, thereby preventing apoptotic elimination via genomic surveillance mechanisms. IKKβ-driven NF-κB also contributes to CAC development in inflammatory cells (macrophages, dendritic cells and/or neutrophils of the lamina propria) where it induces expression of epithelial cell growth factors, such as IL-6.
A fly in the ointment: hepatocyte IKKβ inhibits chemically-induced hepatocellular carcinoma
One of the most common inflammation-linked cancers, thought to be the third leading cause of cancer deaths is HCC 4. Unfortunately, however, the viruses that greatly increase HCC risk, HBV and HCV, cannot be readily propagated in mice and cannot be used for HCC induction in this genetically-manipulatable small mammal. Although liver-targeted oncogenes can induce HCC development in mice 37, 38, we chose to use a chemical carcinogen, diethyl nitrosamine (DEN), to induce highly penetrant HCC in mice whose gene expression profile is very similar to that of aggressive human HCC 39. Nonetheless, we did not anticipate that the carcinogenic action of DEN, which forms a very potent alkylating agent upon metabolic activation in hepatocytes 40, depends on inflammation or inflammatory processes similar to those responsible for HCC induced by chronic viral hepatitis 41, 42. However, because DEN induces DNA damage and thereby activates the p53-dependent cytotoxic stress response and NF-κB can oppose the pro-apoptotic function of p53 43, we expected that mice lacking hepatocyte IKKβ, so-called IkkβΔhep mice 44, will exhibit a more efficient p53-promoted apoptotic response after DEN exposure and should therefore be refractory to HCC induction relative to DEN-treated WT mice. Surprisingly and counterintuitively, IkkβΔhep mice were found to develop many more and faster growing HCCs than control mice 45. However, HCC induced by DEN administration to Ikkβ+/− mice did not display any loss-of-heterozygocity, thereby indicating that IKKβ in hepatocytes does not act as a classical tumor suppressor. Despite the surprising increase in HCC development, IkkβΔhep mice did show the expected increase in DEN-induced apoptosis as well as necrosis. However, due to its highly efficient regenerative capacity, the liver of IkkβΔhep mice contained many more proliferating cells several days after DEN exposure than the liver of similarly treated WT (or IkkβF/F) mice 45. Double-labeling experiments revealed that most of the proliferative hepatocytes were situated next to dying cells – a classic example of compensatory proliferation triggered by hepatocyte death. Additional experiments revealed that neither IKKβ nor NF-κB is a negative regulator of the hepatocyte cell cycle and suggested that the increased compensatory proliferation seen in IkkβΔhep mice is directly due to more hepatocyte loss through apoptosis or necrosis in these animals. NF-κB activation opposes hepatocyte death through a variety of mechanisms, including induction of anti-apoptotic proteins, such as Bcl-XL. DEN, however, induces both apoptosis and necrosis through a complex mechanism that, in addition to DNA damage, includes generation of reactive oxygen species (ROS). Interestingly, NF-κB activation can attenuate ROS accumulation through induction of anti-oxidants such as Mn superoxide dismutase or SOD2 20 and ferritin heavy chain 46. Accordingly, IkkβΔhep mice display elevated ROS accumulation in their hepatocytes after DEN administration relative to similarly treated WT mice 45. ROS contribute to DEN-induced cell death and tumor development, as both are inhibited in response to administration of butylated hydroxyanisole (BHA), a potent antioxidant. Although elevated ROS accumulation may conceivably lead to higher levels of DNA damage and oncogenic mutations, their main effect is enhanced cell death. One of the ways by which ROS promote cell death is through the oxidation of a critical cysteine residue in the catalytic pocket of MAPK phosphatases (MPKs) 20. This results in inhibition of MKP activity and sustained activation of different MAPKs, including JNK1, a critical mediator of hepatocyte death 20, 47. Importantly, inactivation of JNK1 attenuated DEN-induced hepatocyte death and greatly reduced DEN-induced HCC load 47.
These results show that the major role of IKKβ-driven NF-κB in hepatocytes is to provide protection against a variety of cytotoxic challenges and thereby inhibit injury-induced inflammation and compensatory proliferation (Figure 2). These results provide strong biochemical and molecular genetic support to the notion that HCC, whether induced by administration of a carcinogen or by chronic viral hepatitis in humans, is the end result of repetitive liver injury followed by regenerative cell proliferation. High rates of compensatory proliferation increase the likelihood that oncogenic mutations and DNA rearrangements will be fixed in the genome, transmitted to subsequent generations and eventually elicit tumor development. This general mechanism may be applicable to other tissues with inherently low rates of cell division but high capacity for compensatory proliferation.
IKKβ and JNK1 inversely control hepatocyte survival and compensatory proliferation in DEN-treated mice. DEN undergoes metabolic activation in zone 3 hepatocytes resulting in accumulation of ROS, which exert a cytotoxic effect that may be due to sustained JNK activation. DEN can also lead to necrotic cell death through induction of DNA damage and also causes IKKβ and JNK activation through unknown mechamisms. Activation of NF-κB promotes cell survival through different mechamisms, including the upregulation of anti-oxidants, such as SOD2 and FHC that prevent excessive ROS accumulation and prolonged JNK activation, and induction of anti-apoptotic proteins, such as c-FLIP and Bcl-XL. Insufficient activation of NF-κB promotes ROS accumulation, leading to sustained JNK activation and cell death. In addition to its role in cell death, JNK can activate AP-1 transcription factors and enhance cyclin D expression, thereby promoting the proliferation of surviving hepatocytes.
Inflammation and gender control hepatocarcinogenesis
Although inactivation of IKKβ in hepatocytes enhanced development of HCC by increasing the extent of carcinogen-induced liver injury, inactivation of IKKβ in inflammatory cells inhibited the development of HCC, just as it did for CAC 45. These findings strongly suggest that even though DEN-induced hepatocarcinogenesis was thought not to involve inflammation, it is dependent on inflammatory signaling, in this case NF-κB activation in hematopoietic-derived cells, after all. The most critical inflammatory cell for HCC development is likely to be the the Kupffer cell (KC), the resident liver macrophage 45. In support of this proposal, the KC is a major source of IL-6 production in response to DEN administration 45 and IL-6 was found to be essential for DEN-induced hepatocarcinogenesis 48. We have also proposed that necrotic liver injury caused by exposure to DEN or other toxins results in the release of inflammatory mediators that lead to KC activation 45. As the identity of the hypothetical mediators released by dying hepatocytes and the signaling mechanisms through which they operate were nebulous, this proposal remained speculative until recently.
Another striking feature of HCC is its marked gender bias: the worldwide incidence of HCC is 3-5 times higher in males than in females 42. This differential becomes even more staggering when the incidence of HCC is compared in individuals who are younger than 50 years of age, a group encompassing pre-menopausal women. In that group, the incidence of HCC is 7-10 times higher in men than in women. A similar gender bias is seen in rodent models of HCC 49. We noted that following a single DEN dose at 2 weeks of age, the incidence of HCC detected in 8 month old male mice, 100%, is at least 6 times higher than in females of the same age, 15%. This difference declined to 2.5-fold in IkkβΔhep mice because females lacking IKKβ in hepatocytes developed 4 times more HCC than WT females 45. These findings suggested that the refractoriness of female mice to DEN-induced HCC may be due to their relative resistance to DEN-induced hepatic injury, which depends on IKKβ expression. Indeed, we found that male mice develop much more extensive liver injury than females after administration of either DEN or carbon tetrachloride (CCl4), a commonly used liver tumor promoter 48. Although CCl4 is not mutagenic, it undergoes metabolic activation and leads to ROS accumulation in the same type of cell as DEN – the zone 3 hepatocyte. Importantly, DEN or CCl4 administration leads to much higher IL-6 production in male mice than in females and the ablation of IL-6 reduces hepatic injury and almost completely prevents HCC development in male mice 48. Thus, without IL-6, male mice do not develop any more HCCs than female mice. As mentioned above, IL-6 is mainly produced by KC and incubation of KC with proteins released by necrotic hepatocytes induces IL-6 production in a manner dependent on IKKβ activation in KC and is suppressible by estrogen (E2) and selective estrogen receptor α (ERα) agonists.
We speculated that proteins released by necrotic hepatocytes may activate KC via innate immune receptors of the Toll/IL-1R family and therefore examined the dependence of DEN-induced IL-6 production on MyD88, an adaptor protein that plays a critical role in IKK activation by this family of receptors 50. Indeed, KC isolated from Myd88−/− mice produced very little IL-6 upon incubation with proteins released by necrotic hepatocytes. Most importantly, administration of either DEN or CCl4 to Myd88−/− male mice resulted in very little IL-6 production and Myd88−/− males exhibited a remarkable 5-fold decrease in HCC multiplicity relative to similarly treated WT males 48.
These findings suggest the following scheme for induction of HCC by DEN, a mechanism that may apply to other liver carcinogens. DEN undergoes metabolic activation in zone 3 hepatocytes, a few of which may experience oncogenic mutations but many of which die as a result of ROS accumulation. The death of these cells is enhanced in the absence of NF-κB, whose activity curtails ROS accumulation. The dead hepatocytes release normal cellular constituents that lead to activation of KC via TLR/IL-1R family members in a MyD88-dependent manner (Figure 3). Activated KC produce IL-6, which amplifies DEN-induced liver injury through yet-to-be identified mechanisms and stimulates compensatory hepatocyte proliferation probably through activation of the STAT3 transcription factor 48. According to this proposal, DEN-induced hepatocarcinogenesis depends on an inflammatory crosstalk between dying hepatocytes, KC and living hepatocytes that carry oncogenic mutations (Figure 3). The same general mechanism may account for development of human HCC and our results suggest that ERα antagonists that are capable of inhibiting IL-6 production without inducing feminization may be used as chemopreventive agents that block the progression of hepatitis to HCC. A similar preventive effect may be produced by drugs that interfere with IL-6 signaling.
An inflammatory link between hepatocyte necrosis and Kupffer cell activation controls compensatory proliferation and development of hepatocellular carcinoma (HCC). Proteins released by necrotic hepatocytes activate Kupffer cells via members of the Toll/IL-1R family leading to induction of cytokines such as IL-6. IL-6 activates STAT3 in surviving hepatocytes and leads to activation of hepatic stellated cells (HSC) that produce hepatocyte growth factor (HGF). Collectively, these cytokines stimulate the proliferation of surviving hepatocytes. Proliferating hepatocytes that carry oncogenic mutations induced by the mutagenic activity of DEN proceed to form HCC.
IKKα – an enhancer of prostate cancer metastasis
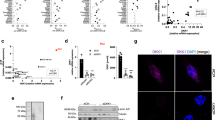
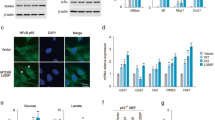
The findings made in the CAC and chemically-induced HCC models underscore the important tumor promoting function of IKKβ in inflammatory cells, but also reveal the complex effects of IKKβ-dependent NF-κB activation in epithelial cells on tumor development. In addition, these studies underscore the critical role played by inflammation in early tumor promotion 51. However, the effect of inflammation is probably not limited to tumor promotion and it may also affect neoplastic progression and the formation of distant site metastases. As neither the CAC nor the HCC models described above are suitable for studying metastasis, we have turned to the TRAMP mouse model of prostate carcinoma (CaP), which mimics many of the aspects of the malignant progression seen in human CaP, including delayed but frequent development of distal organ metastases 52, 53. First, we examined the role of IKKβ in prostate epithelial cells on CaP development in TRAMP mice, in which tumorigenesis is driven by prostate-specific expression of SV40 T antigen, but no effect on either metastatogenesis or tumorigenesis was found (JL Luo et al., in preparation). We therefore have switched to examine the role of IKKα in development and progression of this malignancy. To that end we have used the IkkαAA knockin mouse in which the two serine phosphoacceptor sites responsible for kinase activation were replaced with alanines 54. These mice express normal amounts of an IKKα protein whose kinase activity cannot be turned on in response to upstream stimuli. This mutant retains kinase-independent functions of IKKα, for instance in skin development 29 but lacks those that depend on kinase activation. TRAMP mice that were rendered homozygous for the IkkαAA mutation were found to exhibit decelerated tumor development but eventually all died of primary CaP. However, when analyzed at time of death, IkkαAA/TRAMP mice were found to display much fewer secondary site metastases than WT/TRAMP mice 55. Subsequent analysis verified that IKKα kinase activity was required for metastatogenesis but was not needed for formation of primary tumors and had no effect on their tumorigenic potential, determined by subcutaneous implantation of primary CaP cells. To understand how IKKα controls metastatic activity, we examined expression of 40 known positive and negative regulators of metastasis at different stages of tumor progression in both WT/TRAMP and IkkαAA/TRAMP mice and found that IKKα kinase activity controlled the expression of only a single metastasis regulating gene, coding for metastasis inhibitor maspin 55. Maspin is a serine protease inhibitor originally identified for its anti-metastatic activity in mammary carcinomas 56. At early stages of CaP development, WT/TRAMP and IkkαAA/TRAMP mice express similar amounts of maspin in adenocarcinoma cells, but at later stages of progression, WT/TRAMP mice exhibit loss of maspin in CaP cells and this decline correlates with appearance of secondary site metastases. IkkαAA/TRAMP mice, however, retain high levels of maspin expression in CaP cells throughout tumor progression and correspondingly exhibit very few metastases 55. IKKα was found to control maspin expression at the transcriptional level and this has turned out to require IKKα nuclear translocation. Curiously, IKKα contains a nuclear localization sequence that is not present in IKKβ 30. Whereas normal prostate epithelial cells or early CaP cells contain little, if any, nuclear IKKα, advanced WT CaP cells isolated from mice that exhibit metastases contain significant amounts of nuclear IKKα and most of that form appears to be activated 55. In fact, the amount of nuclear IKKα is directly related to metastatic progression and inversely related to maspin expression not only in TRAMP mice but also in human CaP patients 55. Exactly how nuclear IKKα controls maspin transcription is not fully clear, but chromatin immunoprecipitation experiments indicate that IKKα is recruited to the maspin promoter.
Trying to understand what controls the nuclear translocation of IKKα, we found a good correlation between reduced maspin expression, nuclear accumulation of activated IKKα and the presence of tumor infiltrating T cells and macrophages, which appear only at late stages of tumor progression 55. We also found that advanced prostate tumors contained 50-fold more receptor activator of NF-κB (RANK) ligand (RANKL) mRNA and 20-fold more lymphotoxin α (LTα) mRNA than early tumors, which exhibit little, if any, nuclear IKKα. In vitro, RANKL, which activates NF-κB through a receptor called RANK, a member of the TNF receptor family, induced IKKα nuclear translocation in WT prostate epithelial cells and downregulated maspin expression. Nonetheless, this effect of IKKα was not mediated through NF-κB and activation of IKKβ, which does not translocate to the nucleus, had no effect on maspin expression 55. Importantly, RANKL had no effect on maspin expression in IkkαAA prostate epithelial cells, confirming that downregulation of maspin expression is IKKα-dependent.
These results had outlined a new pathway through which tumor-induced inflammation, whose hallmark is the appearance of infiltrating inflammatory cells within the growing tumor, stimulates metastatic progression in CaP and possibly also in mammary/breast cancer. We propose that accelerated tumor growth results in the necrotic death of a subpopulation of carcinoma cells present within the tumor's core that are being starved for nutrients and/or oxygen. The necrotic cells release normal cellular constituents that recruit and activate inflammatory cells, macrophages and T cells, that produce cytokines that further stimulate tumor growth and angiogenesis. Cytokines, such as RANKL, induce activation and nuclear translocation of IKKα in adjacent carcinoma cells and this results in repression of maspin expression. Eventually, the repressed maspin gene is permanently shut-off through epigenetic silencing. Cells that no longer express maspin gain full metastatic potential and can establish distant site metastases. This model, depicted in Figure 4, provides a good explanation for the long delay associated with metastatic progression. It should be noted, however, that inflammation is likely to stimulate metastatogenesis through additional mechanisms that remain to be identified.
A pathway based on IKKα activation and nuclear translocation controls metastatic progression in prostate cancer. The rapid growth of early prostate adenocarcinomas results in necrotic death of CaP cells located at the tumor's core, which are starved for nutrients and oxygen. Necrotic cells release proteins that lead to recruitment and activation of inflammatory cells. The latter produce cytokines, such as RANKL, that activate IKKα and induce its nuclear translocation. Once a sufficient amount of activated IKKα had accumulated in the nucleus, maspin expression is repressed and eventually is epigenetically silenced. Absence of maspin expression results in metastasis.
Conclusions
Starting with the hypothesis that transcription factor NF-κB and the signaling pathways that control its activity provide a molecular link between inflammation and cancer 15, we obtained ample experimental support for the tumor promoting function of IKKβ and the classical NF-κB pathway in several distinct models of cancer 32, 45, 47, 48, 57. We have also succeeded in identifying a new pathway in which IKKα plays an NF-κB-independent role in the control of metastatogenesis. While some of the different pathways that bridge inflammation and cancer remain to be worked out, the major challenge in the near future is to validate the findings made in mice in human cancer patients and translate them to create novel and improved therapeutic and preventive strategies that will reduce the burden of both primary and metastatic cancer. One advantage of targeting the inflammatory component of tumors is that inflammatory cells are genetically normal and stable and thus are unlikely to develop drug resistance as easily as the genetically unstable carcinoma cells. Nonetheless, anti-inflammatory therapy is likely to be most effective in combination with conventional cytotoxic cancer therapy.
References
Balkwill F, Mantovani A . Inflammation and cancer: back to Virchow? Lancet 2001; 357:539–545.
Kuper H, Adami HO, Trichopoulos D . Infections as a major preventable cause of human cancer. J Intern Med 2000; 248:171–183.
Roder DM . The epidemiology of gastric cancer. Gastric Cancer 2002; 5 Suppl 1:5–11.
Fattovich G, Stroffolini T, Zagni I, Donato F . Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004; 127:S35–S50.
Ekbom A . Risk of cancer in ulcerative colitis. J Gastrointest Surg 1998; 2:312–313.
Moore RJ, Owens DM, Stamp G, et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med 1999; 5:828–831.
Arnott CH, Scott KA, Moore RJ, et al. Tumour necrosis factor-alpha mediates tumour promotion via a PKC alpha- and AP-1-dependent pathway. Oncogene 2002; 21:4728–4738.
Lin EY, Nguyen AV, Russell RG, Pollard JW . Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 2001; 193:727–740.
Pollard JW . Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004; 4:71–78.
Garber K . Aspirin for cancer chemoprevention: still a headache? J Natl Cancer Inst 2004; 96:252–253.
Oshima M, Dinchuk JE, Kargman SL, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 1996; 87:803–809.
Koehne CH, Dubois RN . COX-2 inhibition and colorectal cancer. Semin Oncol 2004; 31:12–21.
Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 2000; 342:1946–1952.
Ghosh S, Karin M . Missing pieces in the NF-κB puzzle. Cell 2002; 109 Suppl:S81–S96.
Karin M, Cao Y, Greten FR, Li Z . W. NF-κB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2002; 2:301–310.
Barnes PJ, Karin M . NF-κB - A pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 1997; 336:1066–1071.
Lin A, Karin M . NF-κB in cancer: a marked target. Semin Cancer Biol 2003; 13:107–14.
Liu ZG, Hu H, Goeddel DV, Karin M . Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis, while NF-κB activation prevents cell death. Cell 1996; 87:565–576.
Wang CY, Mayo MW, Baldwin AS Jr . TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science 1996; 274:784–787.
Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M . Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 2005; 120:649–661.
Zong WX, Thompson CB . Necrotic death as a cell fate. Genes Dev 2006; 20:1–15.
DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M . A cytokine-responsive IkB kinase that activates the transcription factor NF-κB. Nature 1997; 388:548–554.
Rothwarf DM, Karin M . The NF-kappa B activation pathway: a paradigm in information transfer from membrane to nucleus. Sci STKE 1999; RE1.
Makris C, Godfrey VL, Krähn-Senftleben G, et al. Female mice heterozygous for IKK gamma/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol Cell 2000; 5:969–979.
Li ZW, Chu W, Hu Y, et al. The IKKβ subunit of IkB kinase (IKK) is essential for NF-κB activation and prevention of apoptosis. J Exp Med 1999; 189:1839–1845.
Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M . The two faces of IKK and NF-κB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med 2003; 9:575–581.
Hu Y, Baud V, Delhase M, et al. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of the IkB kinase. Science 1999; 284:316–320.
Bonizzi G, Karin M . The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol 2004; 25:280–288.
Hu Y, Baud V, Oga T, Kim KI, Yoshida K, Karin M . IKKα controls formation of the epidermis independently of NF-κB. Nature 2001; 410:710–714.
Sil AK, Maeda S, Sano Y, Roop DR, Karin M . IkB kinase-a acts in the epidermis to control skeletal and craniofacial morphogenesis. Nature 2004; 428:660–664.
Okayasu I, Ohkusa T, Kajiura K, Kanno J, Sakamoto S . Promotion of colorectal neoplasia in experimental murine ulcerative colitis. Gut 1996; 39:87–92.
Greten FR, Eckmann L, Greten TF, et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004; 118:285–296.
Coussens LM, Werb Z . Inflammation and cancer. Nature 2002; 420:860–867.
Balkwill F, Charles KA, Mantovani A . Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005; 7:211–217.
Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I . Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 1999; 8:265–277.
Becker C, Fantini MC, Schramm C, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity 2004; 21:491–501.
Lewis BC, Klimstra DS, Socci ND, Xu S, Koutcher JA, Varmus HE . The absence of p53 promotes metastasis in a novel somatic mouse model for hepatocellular carcinoma. Mol Cell Biol 2005; 25:1228–1237.
Fausto N . Mouse liver tumorigenesis: models, mechanisms, and relevance to human disease. Semin Liver Dis 1999; 19:243–252.
Thorgeirsson SS, Grisham JW . Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet 2002; 31:339–346.
Sarma DS, Rao PM, Rajalakshmi S . Liver tumour promotion by chemicals: models and mechanisms. Cancer Surv 1986; 5:781–798.
Chisari FV . Hepatitis B virus transgenic mice: insights into the virus and the disease. Hepatology 1995; 22:1316–1325.
Bosch FX, Ribes J, Diaz M, Cleries R . Primary liver cancer: worldwide incidence and trends. Gastroenterology 2004; 127:S5–S16.
Tergaonkar V, Pando M, Vafa O, Wahl G, Verma I . p53 stabilization is decreased upon NFkappaB activation: a role for NFkappaB in acquisition of resistance to chemotherapy. Cancer Cell 2002; 1:493–503.
Maeda S, Chang L, Li ZW, Luo JL, Leffert H, Karin M . IKKβ is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFalpha. Immunity 2003; 19:725–737.
Maeda S, Kamata H, Luo JL, Leffert H, Karin M . IKKβ couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 2005; 121:977–990.
Pham CG, Bubici C, Zazzeroni F, et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell 2004; 119:529–542.
Sakurai T, Maeda S, Chang L, Karin M . Loss of hepatic NF-κB activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci USA 2006; 103:10544–10551.
Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007; 317:121–124.
Nakatani T, Roy G, Fujimoto N, Asahara T, Ito A . Sex hormone dependency of diethylnitrosamine-induced liver tumors in mice and chemoprevention by leuprorelin. Jpn J Cancer Res 2001; 92:249–256.
Akira S, Uematsu S, Takeuchi O . Pathogen recognition and innate immunity. Cell 2006; 124:783–801.
Karin M, Greten FR . NF-κB: Linking Inflammation and Immunity to Cancer Development and Progression. Nat Rev Immunol 2005; 5:749–759.
Greenberg NM, DeMayo F, Finegold MJ, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA 1995; 92:3439–3443.
Kaplan-Lefko PJ, Chen TM, Ittmann MM, et al. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate 2003; 55:219–237.
Cao Y, Bonizzi G, Seagroves TN, et al. IKKα provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell 2001; 107:763–775.
Luo JL, Tan W, Ricono JM, et al. Nuclear cytokine activated IKKα controls prostate cancer metastasis by repressing maspin. Nature 2007; 446:690–694.
Zou Z, Anisowicz A, Hendrix MJ, et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science 1994; 263:526–9.
Luo JL, Maeda S, Hsu LC, Yagita H, Karin M . Inhibition of NF-κB in cancer cells converts inflammation- induced tumor growth mediated by TNFα to TRAIL-mediated tumor regression. Cancer Cell 2004; 6:297–305.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karin, M. The IκB kinase – a bridge between inflammation and cancer. Cell Res 18, 334–342 (2008). https://doi.org/10.1038/cr.2008.30
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2008.30
Keywords
This article is cited by
-
Probing the Interface of HIV and Inflammaging
Current HIV/AIDS Reports (2021)
-
Inhibition of IKKβ/NF-κB signaling pathway to improve Dasatinib efficacy in suppression of cisplatin-resistant head and neck squamous cell carcinoma
Cell Death Discovery (2020)
-
HBV-related hepatocarcinogenesis: the role of signalling pathways and innovative ex vivo research models
BMC Cancer (2019)
-
Pharmacological Inhibition of NFκB Reduces Prostate Cancer Related Osteoclastogenesis In Vitro and Osteolysis Ex Vivo
Calcified Tissue International (2019)
-
Chronic diseases, inflammation, and spices: how are they linked?
Journal of Translational Medicine (2018)