Abstract
Here we show that the fate of osteolytic bone metastasis depends on the balance among autophagy, anoikis resistance and ossification, and that the hepatocyte growth factor (HGF) signaling pathway seems to have an important role in orchestrating bone colonization. These findings are consistent with the pathophysiology of bone metastasis that is influenced by the cross-talk of supportive and neoplastic cells through molecular signaling networks. We adopted the strategy to target metastasis and stroma with the use of adenovirally expressed NK4 (AdNK4) and Dasatinib to block HGF/Met axis and Src activity. In human bone metastatic 1833 cells, HGF conferred anoikis resistance via Akt and Src activities and HIF-1α induction, leading to Bim isoforms degradation. When Src and Met activities were inhibited with Dasatinib, the Bim isoforms accumulated conferring anoikis sensitivity. The proviability effect of HGF, under low-nutrient stress condition, was related to a faster autophagy deactivation with respect to HGF plus Dasatinib. In the 1833 xenograft model, AdNK4 switched metastasis vasculature to blood lacunae, increasing HIF-1α in metastasis. The combination of AdNK4 plus Dasatinib gave the most relevant results for mice survival, and the following molecular and cellular changes were found to be responsible. In bone metastasis, we observed a hypoxic condition – marked by HIF-1α – and an autophagy failure – marked by p62 without Beclin-1. Then, osteolytic bone metastases were largely prevented, because of autophagy failure in metastasis and ossification in bone marrow, with osteocalcin deposition. The abnormal repair process was triggered by the dysfunctional autophagy/anoikis interplay. In conclusion, the concomitant blockade of HGF/Met axis and Src activity seemed to induce HIF-1α in metastasis, whereas the bone marrow hypoxic response was reduced. As a consequence, anoikis resistance might be hampered favoring, instead, autophagy failure and neoformation of woven bone trabeculae. Mice survival was, therefore, prolonged by overcoming an escape strategy adopted by metastatic cells by disruption of tumor–stroma coevolution, showing the importance of autophagy inhibition for the therapy of bone metastasis.
Similar content being viewed by others
Main
Systemic signals of primary tumor convert the microenvironment of distant organs into more hospitable sites for metastasis engraftment.1 For example, breast cancer participates in the formation of premetastatic niche in the bone through systemic release of hepatocyte growth factor (HGF).2 We have shown that HGF contributes to bone metastasis plasticity by mediating a metastasis-microenvironment cross-talk via Wnt-β-catenin and Src tyrosine kinase network.2, 3 Therefore, bone metastasis does not rely only on Src autonomous program for adapting to demands imposed by foreign tissue at secondary site.4
The patient stratification according to HGF/Met receptor expression or Met phosphorylation needs further development to become an important component of study design in clinical trials.5 HGF/Met system typically transduces at the plasma membrane through a number of cellular- or membrane-associated kinases, including Src. Also, HGF/Met signaling may operate in nuclei of aggressive mammary cancer cells triggering still elusive transduction pathways, centered on Src nuclear activity, and influencing invasive–metastatic phenotype.2, 6, 7 Rescue of micrometastasis from quiescence and metastatic colonization requires engagement of Src and focal adhesion kinase downstream of β1-integrin pathway: blockade of this extracellular matrix (ECM)-triggered signal cascade is an important strategy for preventing or treating recurrent metastatic disease.8 On the whole, Src inhibition would be relevant as Src family kinases are, on the one hand, involved in regulating the activity of various cell types of the bone marrow stroma,9 and on the other hand are implicated in cancer cell proliferation and survival, and in osteoclast differentiation.4
In osteolytic bone metastasis from breast cancer, disruption of mineralized matrix and detachment of metastatic cells from ECM occur.10 Neoplastic cells under duress owing to ECM detachment might undertake autophagy, although the exact role of non-canonical cell death mechanisms have yet to be definitively determined. Oxidative stress is one of the events consequent to ECM detachment, promoting autophagy.11, 12 Excess stromal production of reactive oxygen species (ROS) drives the onset of antioxidant defense in adjacent cancer cells, protecting them from apoptosis.12 In fact, tumor cells must overcome both anoikis and necroptosis in order to metastasize.11
Reconciling several scientific evidences, autophagy becomes more apparent in late stages as tumor cells cope with microenvironmental stress, encountered during progression and metastasis.13 As autophagy has emerged as one of the survival pathways for tumor cells, there is great interest in inhibiting this process for cancer therapy.14
The aim of the present investigation was to clarify the presence of autophagy in bone metastasis of breast carcinoma, and the critical role of HGF/Met axis influencing the hypoxic response. Very little is known about the role, if any, of hypoxia in regulating anoikis or changes in tissue architecture, in relation to breast cancer progression and treatment. Resistance to detachment-induced anoikis is emerging as a hallmark of metastatic malignancies, mainly because it can ensure anchorage-independent growth and survival during organ colonization. We adopted a strategy to block ligand-activated Met receptor and component(s) of its downstream signaling pathway(s) with NK4, using a bone metastasis model prepared with 1833 clone, derived from MDA-MB231 breast carcinoma cells. NK4 exhibits two distinct biological actions as HGF antagonist and angiogenic inhibitor.5, 15 Also, it prevents lung metastases,16, 17 but does not affect proliferation and apoptosis of carcinoma cells.18 In the adopted combination therapy, we targeted Src activity with Dasatinib (DAS), reasoning that NK4 and DAS might be efficient to impair metastatic cell population escaped via Src from selective pressure exerted by the HGF/Met axis blockade.
The cellular processes of angiogenesis and autophagy/ossification were affected by NK4 alone and NK4 plus DAS, respectively. The combined treatment successfully prolonged mice survival preventing osteolysis through bone formation coupled to defective autophagy. One of the molecular events causing autophagy failure was hypoxia with HIF-1α induction due to NK4 effect. In vitro experiments showed that Src activities, both the nuclear HGF-dependent and the HGF-independent, were inhibited by NK4 plus DAS. These pathways were involved in metastatic cell invasiveness, and in anoikis resistance through Akt activity and HIF-1α induction, as well as Bim degradation.
Results
Blockade of HGF and Src impaired bone metastasis outgrowth
A xenograft model was prepared with 1833/TGL cells, engineerized with a luminescent construct. On the basis of our data on the pivotal role of HGF in bone metastasis,2 and the hypothesis of Gherardi et al.,5 regarding Src-mediated selective cell population escape after HGF/Met blockade, we treated 1833 xenograft mice with adenovirally expressed NK4 (AdNK4) in the presence or absence of DAS (Figure 1). The schedule with multiple AdNK4 injections enhanced circulating NK4 levels, with NK4 access to the bone marrow (Supplementary Figures S1a and b).
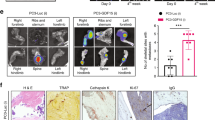
Effects of the inhibitors on bone metastasis outgrowth. (a) Representative bioluminescence imaging (BLI) of two xenograft mice for each experimental group. (b) Absolute quantitative BLI values and (c) fold variations of BLI for hind limbs in the two projections. The fold variations were calculated versus the ME value, considered as 1. ME (n=12); ME+AdNK4 (n=12); DAS (n=8); and AdNK4+DAS (n=8). Averages±S.E.M. *P<0.05, ***P<0.001 versus corresponding ME value; Δ P<0.05 versus AdNK4 value. (d) For all the mice, absolute BLI values for the skeleton were calculated, and percent decreases after treatments are reported. Averages±S.E.M. *P<0.05, **P<0.005 versus corresponding ME value; Δ P<0.05 versus AdNK4 value
We monitored metastatic development in real-time exploiting the bioluminescence of mice bearing bone metastasis (ME), and of ME-treated groups (Figure 1a). The injection efficiency for all the mice was controlled by monitoring the bioluminescence signal 1 h after xenografting (Supplementary Figure S2a). To exclude a potential impact of the AdNK4 and DAS pretreatments on extravasation and homing, with an interference with the evaluation of metastasis growth, we normalized the data of bioluminescence of each animal with the value obtained at 24 h (Supplementary Figure S2a). The bioluminescence value of AdLacZ control mice was similar to that of ME (Supplementary Figure S2b).
As shown in Figures 1b and c, at 13 days from intracardiac cell injection, AdNK4 and DAS alone or in combination reduced metastasis outgrowth to about 90% in both the hind limbs, independent of the projection. At 20 days, the combination of NK4 plus DAS significantly reduced (about 75%) bioluminescence with respect to NK4 alone (about 50% decrease). Considering both the hind limbs and projections, at 20 days DAS was ineffective (Figure 1c). Bioluminescence values all over the skeleton at 20 days were reduced more after the combined treatment of NK4 plus DAS than after the single treatments (Figure 1d).
The combined treatment of NK4 plus DAS delayed death and increased the number of surviving mice by reducing osteolytic bone metastasis
The effectiveness of the combination of AdNK4 plus DAS was further demonstrated by micro-computed tomography (μCT) analysis, showing that osteolysis was largely prevented all over the skeleton (Figure 2a).
Effects of the inhibitors on osteolysis and mice survival. (a) Representative three-dimensional reconstruction of μCT images. Three xenograft mice per group were analyzed with similar results. The arrows indicate osteolysis, largely prevented after AdNK4 plus DAS. (b) Survival curve on Kaplan–Meier plots of the data from treated and untreated ME. (c) Effects of the various treatments on the number of surviving mice. (d) ELISA of circulating cytokines in ME injected or not with AdNK4; n=8 per group. Averages±S.E.M. For plasma NK4, *P<0.05 versus 15-day value. For HGF, normal control value in plasma was 0.02 fg/ml. (e, f) Absolute BLI values; n=8 per group. Averages±S.E.M. *P<0.05 versus DAS value
ME mice died at 26 days on an average, and the treatments positively but differentially influenced survival (Figure 2b). All DAS-treated animals died between days 32 and 33, whereas NK4 treatment delayed death further by 4 days (Figure 2c). Thus, with respect to ME animals, which by day 26 were all dead, NK4 promoted survival as about 25% of the mice lived up to 36 days. The combined AdNK4 plus DAS treatment was even more effective because 50% of the mice survived until 35 days and died thereafter (day 38), that is 12 days later than ME. The treatments did not affect body weight with respect to ME (Supplementary Figure S2c).
Measurement of HGF and NK4 in the plasma (Figure 2d) showed similar HGF levels in ME between 15 and 20 days, despite bone metastasis increase in size and volume. It is reasonable to assume, therefore, that HGF was primarily produced by the host bone stroma rather than by metastatic cells. Cellular HGF may be secreted in the plasma or used locally.2 NK4 plasma levels progressively decreased from day 15 to day 30.
As DAS and NK4 survival curves underwent inversion around day 30 (Figure 2b), we compared at this time the effects of different treatments on bioluminescence. As shown in Figures 2e and f, DAS alone seemed less effective than AdNK4 plus DAS, especially considering the total burden.
Cellular processes and molecular markers affected in vivo by NK4 plus DAS versus NK4
Considering that in any case AdNK4 plus DAS prolonged mice survival, with respect to AdNK4 alone, notwithstanding similar bioluminescence signals at 30 days, in the following experiments we tried to give an explanation examining cellular processes possibly affected by AdNK4 plus DAS versus the single treatments (Figures 3, 4, 5).
Analysis of autophagy markers in the ME mice groups exposed to AdNK4 with or without DAS. (a) Representative hematoxylin and eosin (H&E) staining of bone metastasis tissue from the different mice groups. Five serial sections were examined for each specimen from three xenograft mice with similar results. (b) Representative immunostaining images for Beclin-1 and p62; magnifications are reported in the insets. The experiments have been repeated on five serial sections for each specimen from three xenograft mice with similar results. For (a) and (b), me, metastasis; bv, blood vessel; bo, bone; bm, bone marrow; bl, blood lacunae
Effect of AdNK4 plus DAS on osseous formation in the bone marrow of xenograft mice. (a) Representative hematoxylin and eosin (H&E) staining of bone marrow is shown; arrows indicate woven bone. Five serial sections were examined for each specimen from three xenograft mice with similar results. (b) Representative immunostaining images for osteocalcin; magnifications are reported in the insets. Arrows indicate woven bone. The experiments have been repeated on five serial sections for each specimen from three xenograft mice with similar results. For (a) and (b), me, metastasis; bo, bone; bm, bone marrow. (c) A Scheme of osseous formation after AdNK4 plus DAS treatment is shown
Analysis of vascular changes and hypoxic consequences caused by AdNK4 alone or combined with DAS in xenograft mice. Representative immunostaining images: (a) for CD31 and (b) for HIF-1α; magnifications are reported in the insets. Arrows indicate endothelial cells. The experiments have been repeated on five serial sections for each specimen from three xenograft mice with similar results. me, metastasis; bl, blood lacunae; bv, blood vessel; bo, bone; bm, bone marrow
Canonical H&E staining was performed (Figure 3a). As reported,2 ME bone marrow cavity of hind limbs was extensively colonized by metastasis already at 25 days from 1833 cell injection. NK4 (36 days) affected metastasis blood vessels that switched to wide blood lacunae. AdNK4 plus DAS (36 days) gave remarkable alterations of tissue architecture, because of extensive intercellular void spaces, suggesting autophagy dysfunction.14
To address this latter possibility, we analyzed the expression of autophagy-related proteins19 (Figure 3b). Beclin-1 was expressed throughout bone metastatic cells, including nuclei, and a strong Beclin-1 signal was observed also after AdNK4 treatment. After AdNK4 plus DAS, however, Beclin-1 disappeared, consistent with autophagy failure. The p62 pattern was opposite with respect to that of Beclin-1: absent in ME, and in ME plus AdNK4, p62 signal appeared after AdNK4 plus DAS treatment with prevalent nuclear positivity. Ubiquitin-containing p62 bodies are targeted by autophagy.20 Negative controls performed without specific antibodies did not give positive staining.
An elevated Beclin-1 signal, marker of autophagy, was also observed in metastasis stroma in the presence or absence of AdNK4 (Supplementary Figure S3).
As shown in Figures 4a and b, AdNK4 plus DAS caused a surprising effect also at the medullary cavity level, where H&E and osteocalcin stainings evidenced trabeculae of woven bone similar to that observed in primary ossification. Of note, osteocalcin signal was present in metastatic cells, possibly due to osteomimicry.21 Negative controls performed without specific antibodies did not give positive staining. A scheme accounting for bone matrix organization at metastatic site is shown (Figure 4c). Altogether, the combination NK4 plus DAS appeared to interfere with metastasis autophagy, and caused woven bone deposition. The latter process might counteract the metastasis-driven alteration(s) of osseous physiological turnover.
Even if the AdNK4 group did not undergo autophagy failure, differently from AdNK4 plus DAS group (Figure 3), the latter group at 30 days still presented bioluminescence signal in metastases, at an intensity similar to that of AdNK4-treated animals (Figures 2e and f). Autophagy failure in the AdNK4 plus DAS group determines an intercellular discontinuity, but the metastatic cells seemed to maintain the luciferase construct during the time of observation. Growth was likely to be arrested, but the neoplastic cells were not shed from metastasis at 30 days in both AdNK4 plus DAS and AdNK4 groups, leading to similar bioluminescence all over the skeleton.
To further clarify the cellular and molecular mechanisms involved, immunohistochemistry (IHC) was performed with anti-CD31, to evaluate the structure of blood vessels (Figure 5a). In metastatic tissue (me) of ME group, the blood vessels were imperfect but appeared still delimited by endothelial cells, marked by CD31. After AdNK4 injections in ME, we observed blood lacunae without the endothelial lining.
We then evaluated the expression of HIF-1α, which is a critical player in the cellular response to hypoxia (Figure 5b). In the ME group, HIF-1α was principally expressed in the bone marrow, whereas metastasis showed a lower HIF-1α signal throughout the cell, localizing mostly in the cytosol. AdNK4 alone or in combination with DAS modified HIF-1α pattern: the HIF-1α signal was higher in bone metastasis than in bone marrow. In the ME plus AdNK4 group, the HIF-1α signal in bone marrow appeared only in the endothelial lining of blood vessels.
Negative controls performed in the absence of primary antibody did not show specific signals (Figures 5a and b).
Our data regarding the markers of autophagy and hypoxia indicated that: (1) positivity for p62/HIF-1α, without Beclin-1, meant autophagy failure in AdNK4 plus DAS group; (2) HIF-1α/Beclin-1 positivity without p62 might suggest a hypoxic condition due to NK4-dependent blood lacunae formation, with possible loss of anoikis resistance (Supplementary Figure S4).
Effects of NK4 and DAS in vitro on signaling pathways and invasiveness triggered by HGF
In 1833 cells exposed to HGF, we explored the signaling pathways that might be affected by the treatments. In Figure 6, HGF-dependent time courses of Met phosphorylation at the catalytic site and of the canonical downstream pathways are reported. HGF rapidly (within 5 min) and strongly (hundred folds) enhanced the ratios phospho-Met (pMet)/Met and phospho-Akt (pAkt)/Akt. Then, these ratios underwent a diminution, but persisted at high levels for almost 60 min. At earlier times after HGF treatment, Src and ERK1/2 phosphorylation tripled and doubled, respectively.
Time courses of Met and of signal protein kinases, as well as their phosphorylation state after HGF exposure. Representative images of western blots performed with total protein extracts are shown. The histograms show the fold variations of the ratios of phosphorylated/unphosphorylated proteins, calculated after densitometric evaluation. The data are shown as Averages±SEM of three independent experiments. *P<0.05, **P<0.005, ***P<0.001 versus starved untreated cells
As shown in Figure 7a, in total extracts NK4 treatment abrogated phosphorylative activation of Met and Akt observed 5 min after HGF exposure without significantly affecting Src and ERK1/2 phosphorylation. Conversely, DAS treatment reduced HGF-mediated activation of Met, Akt and ERK1/2, but completely abolished Src activation either in the presence or absence of HGF. As expected, the combined NK4 plus DAS treatment abrogated the HGF-dependent activation of all the kinases studied. These results indicate that Src activation contributes only partially to the HGF/Met-mediated transduction pathways, triggered at the plasma membrane level.
Cell signaling and migration after exposure to HGF in the presence or the absence of the inhibitors. (a) Representative images of western blots performed with total protein extracts are shown. We report the fold variations of the ratios of phosphorylated/unphosphorylated proteins, calculated after densitometric evaluation. Vinculin was used for normalization. Averages±S.E.M. of three independent experiments. *P<0.05, **P<0.005, ***P<0.001 versus starved untreated cells. Δ P<0.05, ΔΔΔ P<0.001 versus HGF-treated cells. (b) Nuclear extracts from treated cells were immunoprecipitated with anti-Src antibody: western blots and histograms with fold variations of pSrc/Src values are shown. The experiments were repeated three times with similar results. **P<0.005 versus starvation value; ΔΔ P<0.005, ΔΔΔ P<0.001 versus HGF value. (c) Nuclear extracts from transfected cells were analyzed by western blot, which was hybridized with anti-HGF antibody. B23 was used for normalization. The experiments were repeated three times with similar results. (d) Matrigel invasion assay was performed. Averages±S.E.M. of three independent experiments performed in triplicate. *P<0.05 versus starved untreated cells. Δ P<0.05, ΔΔ P<0.005 versus HGF-treated cells. (e) Schematic representation of in vitro data
To deepen the knowledge of the role played by HGF in Src activation, we studied the effect of AdNK4 at the nuclear level (Figure 7b). Src phosphorylation was evaluated after immunoprecipitation of Src from nuclear extracts of 30-min HGF-treated cells. HGF tripled phospho-Src (pSrc), which was largely impaired by NK4 or DAS exposure. In nuclear extracts of 1833 cells infected with AdNK4 (Figure 7c), we detected HGF and NK4, the latter being absent in AdLacZ-control cells.
To assess some of the biological consequences of NK4 plus DAS treatment, we examined the invasiveness of 1833 cells through Matrigel. As shown in Figure 7d, NK4 reduced the basal cell motility to about 30%, while preventing the HGF-induced invasiveness to about 50%. DAS, either alone or combined with NK4, decreased by more than 90% the HGF-dependent cell invasion.
Figure 7e summarizes a model whereby NK4 exerted an inhibitory effect on Src phosphorylation at the nuclear level, whereas the blockade of HGF/Met axis at the plasma membrane reduced pAkt. This led us to suppose the existence of an alternative HGF-independent pathway for Src activation. DAS was effective in inhibiting pSrc in the presence or absence of HGF.
Central role of Src in anoikis resistance of 1833 cells exposed to HGF, and interaction with autophagy
In vivo in ME xenograft model, microenvironment stimuli like HGF maintain metastatic cell survival and metastatic tissue architecture.2 Thus, we examined whether HGF was involved in viability and anoikis resistance of 1833 cells, as well as the role played by Src and Akt.
In 1833 cells grown on an antiadhesive substratum, HGF pretreatment enhanced viability that was largely dependent on Akt activity (Supplementary Figure S5a). The time course of Akt phosphorylation was evaluated at earlier and later times after HGF (Supplementary Figure S5b).
Under the non-adhesive condition, we also examined the effect of 36-h pretreatment with HGF in the presence or absence of DAS: at various times thereafter, we evaluated cell viability with MTT assay, and Beclin-1 steady-state protein levels (Figures 8a and b). In these experiments, we intended to correlate stress, due to 48 h starvation, and autophagy under our non-adhesive experimental conditions. At the end of the pretreatment, cell viability was 90% (considered as 1); HGF pretreatment unaffected viability until 24 h, while decreasing thereafter, and the concomitant DAS exposure strongly counteracted the prosurvival effect of HGF starting from 12 h (40% decrease) until 72 h (95% decrease) (Figure 8a). Beclin-1 was downregulated by HGF pretreatment faster than by HGF plus DAS pretreatment (Figure 8b). The stress condition, consisting in fetal bovine serum deprivation, was tested on Beclin-1 localization and expression by immunofluorescence (Supplementary Figure S5c).
DAS affects anoikis resistance due to HGF. (a) Starved cells, pretreated with HGF in the presence or absence of DAS for 36 h, were seeded on poly-HEMA, an anchorage-independent condition. Then, cell viability was evaluated at various times by MTT assay. The experiments were repeated three times with similar results. The data are shown as averages±S.E.M *P<0.05, **P<0.005, ***P<0.001 versus the value of viable cells after 36 h HGF, considered as 1. (b) Starved cells, pretreated with HGF in the presence or absence of DAS for 36 h, were seeded on poly-HEMA. Then, the cells were harvested at various times, and total proteins were extracted and analyzed by western blots. Representative images of experiments performed three times are reported. Vinculin was used for normalization. The numbers at the bottom indicate the fold variations, relative to time 0 (the end of pretreatment), considered as 1. (c) Representative hematoxylin and eosin (H&E) staining of bone metastasis tissue. Five serial sections were examined for each specimen from three xenograft mice with similar results. me, metastasis; bv, blood vessel; bo, bone; bm, bone marrow. (d) Western blots of total and nuclear extracts were performed in triplicate, and representative images are shown. Vinculin and B23 were used for normalization. The numbers at the bottom indicate the fold variations of the three Bim isoforms, each versus the relative starvation value, taken as 1. For the other proteins, the fold variations were calculated versus the relative starvation value, taken as 1. (e) Schematic representation of signaling pathways downstream of HGF/Met involved in anoikis resistance, including the role of ROS on Src activation and of the latter on HIF-1α induction.37 DAS relieved anoikis resistance due to HGF
In the xenograft model, DAS (33 days) had marginal or no effects on bone metastatic tissue architecture, examined by H&E staining (Figure 8c).
As shown in Figure 8d, under HGF with or without DAS the marker of anoikis Bim was analyzed, and different isoforms were detected. BimEL, strong in control-starved cells, was degraded under HGF. Also BimL and BimS were less expressed after HGF exposure. DAS addition led to progressive BimEL, BimL and BimS accumulation. HGF biphasically enhanced Akt phosphorylation, consistent with the time-course data of Supplementary Figure S5b, that was almost completely prevented by DAS cotreatment. HIF-1α was studied in extracts from nuclei, where it has its function,22 and we observed HIF-1α induction by HGF (Figure 8d). HIF-1α is a key player of hypoxia-induced anoikis.23, 24
Figure 8e summarizes the signaling pathways downstream of HGF/Met, including a small ROS burst involved in anoikis resistance, and the role of DAS in anoikis triggering.
Discussion
For the first time we have shown the occurrence of autophagy in the human bone metastasis xenograft model, evidenced through the opposite expression of the markers Beclin-1 and p62, consistent with adaptation to stress posed by foreign tissue.25 As p62 is an autophagy substrate, defects of autophagy cause p62 accumulation.14 The role of autophagy during cancer progression depends on tumor type, context and stage. In bone metastasis from breast cancer, and in supportive fibroblasts, autophagy seemed to function as a survival mechanism under the various forms of microenvironment duress. In fact, osteolysis and ECM detachment occurred, and the remarkable HIF-1α signal also indicated hypoxic changes.23 The microenvironmental features stimulate several adaptative responses in metastatic cells, such as activation of antioxidative transcription factors HIF-1 and NF-κB,22, 26 and the release of antiapoptotic stimuli such as HGF.27, 28 HGF is critical both locally in the bone microenvironment,2, 23 due to paracrine (stroma cells) and autocrine (metastasis) production, and systemically because of the elevated levels in the bloodstream. Competitive inhibition of HGF ligand with NK4 might target Met receptor function on more than one cell type, such as metastasis and bone cells. Osteoclasts are also responsive to HGF.29
The therapeutic strategy, consisting in the combined treatment of AdNK4 plus DAS, inhibited in bone metastatic tissue both the HGF/Met signaling cascade and the total Src activity, hampering escape strategies of metastatic cells through Src-dependent rescue pathway(s). Mice survival was prolonged for about 12 days, and osteolytic metastases were prevented all over the skeleton.
The efficacy of NK4 plus DAS strategy against osteolytic metastasis might be explained by the different cellular processes affected. In the metastasis bulk, NK4 plus DAS caused autophagy failure, and woven bone was observed in the bone marrow. The rescue processes of the bone that resembled primary ossification, with osteocalcin deposition in association with defective autophagy, could be important for prolonging mice survival. The higher effectiveness of the combined treatment of NK4 plus DAS than NK4 alone might be related, in any case, to the hypoxic condition in metastasis versus stroma, implicated in autophagy failure.
One of the principal findings of our study was, therefore, the abnormal repair effect of the combined treatment. Enhancement of osteocalcin, a non-collagenous bone matrix protein, created a circuit to impair the outgrowth of bone metastasis also impinging on autophagy. Thus, the cancer tissue organization field theory, related to ECM composition,30 might be applied. Our hypothesis is that the therapeutic impairment of paracrine tumor–stroma cell interaction promoted pro-osteogenic changes. Arrested metastatic cells cannot disturb anymore the differentiation of osteoprogenitors.
We propose that the blockade of the angiogenic role of HGF and of Src multiple functions, might affect various key steps for colonization. The combination of NK4 plus DAS not only affected the network of events, responsible for HGF-triggered chemoinvasion, but was also likely to impact on exit from dormancy, which is known to require Src activity,31 and on stromal autophagy, a new protective mechanism for bone metastasis, besides allowing escape from antiangiogenic therapies.12 Altogether, the combined treatment of NK4 plus DAS was more effective than the single treatments.
Notably, our results are the first to report a therapeutic function of NK4 against breast cancer bone metastasis, prolonging mice survival, when compared with DAS. NK4 induced the formation of blood lacunae lacking endothelial lining, at a difference with metastatic blood vessels, and might prevent metastasis adhesion. NK4 is known to impair endothelial cells by binding to perlecan and inhibiting, therefore, the fibronectin assembly.32 In ME plus AdNK4 group, the blood lacunae increased HIF-1α/hypoxia in metastasis, whereas HIF-1α disappeared in the stroma/bone marrow. We cannot exclude that the stroma protective function towards anoikis in metastasis became defective, Beclin-1 persisting elevated.
High doses of ROS under hypoxia maintain elevated expression of HIF-1α, and are also responsible for Src activity inhibition and mitochondria dysfunction.33 However, different roles are played by a small burst of ROS downstream of growth factor receptors, such as Met stimulated by HGF.27, 34 ROS at low levels might be important for anoikis resistance in 1833 cells exposed to HGF.
The existence of multiple signaling pathways regulating anoikis is likely to be critical for neoplastic cell survival. Bim exists in various isoforms, generated by alternative splicing. In starved 1833 cells, known to produce more transforming growth factor-β than HGF, with pro- and antiapoptotic functions,23 BimEL and BimL were elevated. Transforming growth factor-β increases Bim expression levels and enhances the proapoptotic function by downregulating Akt and ERKs.35 BimL supports acidification of lysosomes that may associate with autophagyc vesicles.36 The 1833 cells were susceptible to autophagy under low nutrient (stress) condition.
In the present paper, we show that the anoikis function of Bim was regulated by Akt activity downstream of HGF. In response to HGF, persistent Akt phosphorylation seemed to be involved in the post-transcriptional regulation of Bim via ubiquitination and proteasomal degradation, attenuating its apoptotic function. In addition, the prosurvival role of HGF did not implicate autophagy under stress and non-adhesive conditions.
Notwithstanding the known acquisition of resistance to anoikis of carcinoma cells during metastatic progression, and suppression of anoikis under hypoxic conditions in an HIF-1α/Src-dependent manner,33, 37, 38 our results allow new insights into the etiology of the molecular mechanisms granting anoikis resistance of bone metastatic cells, in the absence of proper adhesion and under HGF stimulus. Concomitant DAS exposure of 1833 cells, through suppression of pMet/Src/Akt pathways, converging on Bim phosphorylation, abolished its degradation finally leading to the accumulation of BimS, principal mediator of anoikis. Met activity is known to be controlled by phosphorylation also via Src.39
HGF-dependent Src activity was observed only in nuclei, consistent with previous data.2 DAS prevented Src activation dependent or independent on HGF/Met axis. For example, Src activity is controlled by prostaglandin E2 downstream of cyclooxygenase 2, critical enzyme in 1833 cells.23, 40
DAS delayed the growth of metastasis during the first period of observation of the xenograft model: eventually and suddenly the animals died overcoming Src inhibition. The present results confirm and extend previous studies in which DAS triggers apoptosis of 1833 cells implanted in the marrow of tibia being, however, ineffective on homing and on the proportion of proliferating (Ki67+) cells, as well as on osteoclasts located at the tumor–bone matrix interface.4 Similarly, Src dominant-negative or DAS treatment impairs bone metastasis after parental MDA-MB231 cells implant in the tibia.41
In conclusion, the demonstration that tumor cells – surviving to antiangiogenic therapies through autophagy – exhibit increased invasiveness fits with our data obtained with NK4 in the presence of HGF in vitro (Figure 7 and Supplementary Figure S4) and in vivo (Figure 3). Autophagy inhibition could prevent invasion, occurring after antiangiogenic therapy, by disrupting it at an early stage, which may be more effective than targeting invasion directly.42 Akt activity itself downstream of HGF/Met is implicated in tumor angiogenesis by increasing vascular endothelial growth factor secretion, and by mediating the expression of nitric oxide and angiopoietins.43 The meshwork of molecular and cellular mechanisms, underlying tumor–stroma interaction in bone metastasis, may be successfully targeted with therapeutic interventions, exploited to prevent late recurrence in breast cancer patients by inhibiting autophagy. On the basis of preclinical evidence, autophagy inhibition is currently being investigated as a way of modulating the response to cancer therapies in patients.
Materials and Methods
Materials
Recombinant human HGF was from R&D System (Abingdon, UK). The anti-Bim (H-191) antibody, and the antibody used for Src(SRC2) immunoprecipitation were from Santa Cruz Biotechnology (Santa Cruz, CA, USA): anti-c-Src (clone GD11) and anti-pSrc (clone 9A6) for the corresponding immunoblotting were from Upstate Biotechnology (Lake Placid, NY, USA). Anti-phospho-Met(Tyr1234/1235) and the antibodies for Met, Akt, pAkt, Src, pSrc, ERK1/2, pERK1/2 were from Cell Signaling Technology (Beverly, MA, USA). Anti-HIF-1α (clone 54) for western blot was from BD Transduction Laboratories (Lexington, KY, USA). Anti-Beclin-1 and anti-HIF-1α (used for IHC) were from Novus Biologicals (Cambridge, UK). Anti-CD31, anti-osteocalcin and anti-SQSTM1/p62 were from Abcam (Cambridge, UK). DAS was from Selleck Chemicals (Munich, Germany). Poly-hydroxyethyl methacrylate (poly-HEMA) and MTT assay kit were from Sigma-Aldrich (St. Louis, MO, USA).
Cell cultures and treatments
The 1833 bone metastatic clone, derived from MDA-MB231 breast carcinoma cells, and the 1833 cells retrovirally transfected with HSV1-tk/GFP/firefly luciferase (1833/TGL)44 were a gift from Joan Massagué (Memorial Sloan-Kettering Cancer Center, New York, NY, USA).
The 1833 starved cells, with or without 100 ng/ml HGF treatment,2 were exposed or not to 10 μg/ml NK4 for 4 h,17 or were pretreated for 36 h with 0.1 μM DAS. DAS alone, or in combination with NK4, was added two times to the cells.45 Cell infection with AdCMV-NK4 (AdNK4) or AdCMV-LacZ (AdLacZ) (50 MOI) was performed for 48 h.46 All these cells were used to prepare protein extracts.
Some cells underwent starvation for 48 h, and during the last 36 h were exposed to HGF in the presence or absence of DAS; when indicated, transfection with 2 μg/ml of the expression vector for Akt dominant negative was also performed (Jim Woodgett, Mount Sinai Hospital, Toronto, Canada). Then, the cells were plated onto poly-HEMA-coated wells at 1 × 105 cells per cm2 for anchorage-independent growth.47 At the end of the 36-h pretreatment, the number of viable cells was determined by trypan blue exclusion and counting. The MTT assay was performed at various times thereafter, following the manufacturer’s protocol.48 Cell suspension in multiwell was added MTT at a final concentration of 0.5 mg/ml for 3 h, and the resulting formazan crystals were solubilized in DMSO. Optical density was measured at 490 nm, with background subtraction at 630 nm. At the same times, the cells were collected and total protein extracts were prepared and analyzed by western blot.
Xenograft model
Animal studies were carried out according to the Institutional Guide for Care and Use of Laboratory Animals, and with International laws. The 1833/TGL cells were injected in the heart of nu/nu mice;2 some cells were pretreated with 0.1 μM DAS for 24 h. For details of intracardiac cell injection, see Supplementary Experimental procedures. The animals (n=46) were divided into five groups: 1833/TGL injected (ME, n=12); ME+AdNK4 (n=12) and ME+AdLacZ (n=6); ME+DAS (n=8); and ME+AdNK4+DAS (n=8). The adenoviral vectors AdNK4 and AdLacZ (control) (109 PFU per mouse)16 were administered intravenously 1 day before 1833/TGL cells, and then every 5 days. AdNK4 is a replication-deficient adenovirus vector, based on human adenovirus type 5 with E1 and E3 deletions, that expresses human NK4 gene under the transcriptional control of the cytomegalovirus early promoter and enhancer.49 The recombinant virus was grown in the HEK293 cells, and then purified and concentrated with Adeno-X Maxi Purification Kit (Takara Bio Europe/Clontech, Saint-Germain-en-Laye, France). Titers of viral stocks were determined by plaque assays using the HEK293 cells. AdLacZ adenoviral vector, which expresses the LacZ gene, was prepared as reported above, and was used as control vector. The DAS group of animals received DAS (50 mg/kg per os) concomitantly with the intacardiac injection of DAS-pretreated 1833/TGL cells, and daily thereafter.45 Firefly D-luciferin (150 mg/kg) was given intraperitoneally under anesthesia, and metastasis formation was monitored at Transgenic-Operative Products s.r.l. (Lodi, Italy) as luminescence signals by Optical Imaging, using Xenogen IVIS 200 System (Caliper Life Sciences, Hopkinton, MA, USA), and photon emission was quantified with Living-Image Software (Caliper Life Sciences). Acquisition time for bioluminescence at the beginning of observation time points (1 and 24 h) was 5 min; for the following observations, the acquisition time was reduced to 1 min in accordance with signal strength, to avoid saturation. Ventral and dorsal projections were examined. For normalization of the data, regions of interest (ROI) all over the skeleton were defined, and the corresponding bioluminescence values were evaluated at 24 h in ME exposed or not to the treatments. We verified that the different groups had similar bioluminescence value at ROI level. Then, bioluminescence signals of subsequent images for each animal were normalized to the signal value obtained at 24 h. This procedure avoids variations due to homing and extravasation. Osteolysis was monitored by μCT using a Triumph GE, a fully integrated molecular Imaging System PET/SPEC/CT, at Ephoran-MultiImaging Solutions (Bioparco Colleretto di Giacosa, Torino, Italy). Three-dimensional images were reconstructed using Slicer 3D software.
ELISA
HGF and NK4 were evaluated in the plasma of AdNK4 injected xenograft mice by Quantikine Immunoassay (R&D System).
Western blot, immunoprecipitation and Matrigel invasion assays
Total (100 μg) and nuclear (50 μg) protein extracts were used for western blots. The antibodies and their dilutions were as follows: anti-HGF (1:200); anti-Met, anti-pMet, anti-Src, anti-p-Src, anti-Akt, anti-pAkt, anti-ERK1/2, anti-pERK1/2 (1:1000), anti-Bim (2 μg/ml), anti-HIF-1α (1:350) and anti-Beclin-1 (1:500). For immunoprecipitation, 500 μg of nuclear proteins from 30 min HGF-treated cells, in the presence or absence of the inhibitors, were probed with 3 μg of anti-Src antibody, followed by immunoblotting with the antibody for anti-Src (1 μg/ml) or anti-pSrc (2 μg/ml).26 For invasion assay, treated cells were seeded in the upper chambers of BD Biocoat Cellware (Becton Dickinson Labware); HGF was present for 20 h in the lower chambers during the incubation. Ten fields under × 200 magnification were randomly selected, and the number of the cells in each field was counted and averaged.3
Immunohistochemistry
Femora and tibiae from the five groups of xenograft mice were fixed and decalcified,2 except for CD31 staining, performed on frozen sections fixed with 1% paraformaldehyde.15 Serial sections were stained with H&E or were immunostained with the following antibodies: anti-Beclin-1 (1:400), anti-SQSTM1/p62 (1:150), anti-osteocalcin (5 μg/ml), anti-CD31 (1:50) and anti-HIF-1α (1:100). Negative controls were performed without the specific antibody.
Immunofluorescence assay
The cells (8 × 104 per well) on coverslips were starved for various times or were exposed to 10% fetal bovine serum for 72 h. After fixation with 4% paraformaldehyde, reactions were performed with anti-Beclin-1 antibody (1:100), followed by Alexa Fluor 568 reaction. Nuclei were stained with DAPI. Cells were observed at × 400 magnification under fluorescence microscopy.23
Statistical analysis
The statistical analysis was performed by analysis of variance, with the exception of the survival data of xenograft mice, which were analyzed by Kaplan–Meier method and the log-rank (Mantel–Cox) test. P<0.05 was considered significant.
Change history
09 June 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41419-022-04991-7
Abbreviations
- AdNK4:
-
adenovirally expressed NK4
- BLI:
-
bioluminescence imaging
- DAS:
-
Dasatinib
- ECM:
-
extracellular matrix
- HGF:
-
hepatocyte growth factor
- IHC:
-
immunohistochemistry
- ME:
-
mice bearing bone metastasis
- μCT:
-
micro-computed tomography
- ROS:
-
reactive oxygen species
References
Psaila B, Lyden D . The metastatic niche: adapting to the foreign soil. Nat Rev Cancer 2009; 9: 285–293.
Previdi S, Maroni P, Matteucci E, Broggini M, Bendinelli P, Desiderio MA . Interaction between human-breast cancer metastasis and bone microenvironment through activated hepatocyte growth factor/Met and β-catenin/Wnt pathways. Eur J Cancer 2010; 46: 1679–1691.
Matteucci E, Maroni P, Luzzati A, Perrucchini G, Bendinelli P, Desiderio MA . Bone metastatic process of breast cancer involves methylation state affecting E-cadherin expression through TAZ and WWOX nuclear effectors. Eur J Cancer 2013; 49: 231–244.
Zhang XH, Wang Q, Gerald W, Hudis L, Norton M, Smid JA et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 2009; 16: 67–78.
Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G . Targeting Met in cancer: rationale and progress. Nat Rev Cancer 2012; 12: 89–103.
Bendinelli P, Maroni P, Matteucci E, Desiderio MA . Comparative role of acetylation along c-SRC/ETS1 signaling pathway in bone metastatic and invasive mammary cell phenotypes. Biochim Biophys Acta 2011; 1813: 1767–1776.
Matteucci E, Bendinelli P, Desiderio MA . Nuclear localization of active HGF receptor Met in aggressive MDA-MB231 breast carcinoma cells. Carcinogenesis 2009; 30: 937–945.
Barkan D, El Touny LH, Michalowski AM, Smith JA, Chu I, Davis AS et al. Metastatic growth from dormant cells induced by a Col-I-enriched fibrotic environment. Cancer Res 2010; 70: 5706–5716.
Araujo J, Logothetis C . Targeting Src in metastatic bone disease. Int J Cancer 2009; 124: 1–6.
Weilbaecher KN, Guise TA, McCauley LK . Cancer to bone: a fatal attraction. Nat Rev Cancer 2011; 11: 411–425.
Buchheit CL, Rayavarapu RR, Schafer ZT . The regulation of cancer cell death and metabolism by extracellular matrix attachment. Semin Cell Dev Biol 2012; 23: 402–411.
Lisanti MP, Martinez-Outschoorn UE, Chiavarina B, Pavlides S, Whitaker-Menezes D, Tsirigos A et al. Understanding the ‘lethal’ drivers of tumor–stroma co-evolution: emerging role(s) for hypoxia, oxidative stress and autophagy/mitophagy in the tumor microenvironment. Cancer Biol Ther 2010; 10: 537–542.
Roy S, Debnath J . Autophagy and tumorigenesis. Semin Immunopathol 2010; 32: 383–396.
White E . Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer 2012; 12: 401–410.
Suzuki Y, Sakai K, Ueki J, Xu Q, Nakamura T, Shimada H et al. Inhibition of Met/HGF receptor and angiogenesis by NK4 leads to suppression of tumor growth and migration in malignant pleural mesothelioma. Int J Cancer 2010; 127: 1948–1957.
Kishi Y, Kuba K, Nakamura T, Wen J, Suzuki Y, Mizuno S et al. Systemic NK4 gene therapy inhibits tumor growth and metastasis of melanoma and lung carcinoma in syngeneic mouse tumor models. Cancer Sci 2009; 100: 1351–1358.
Kuba K, Matsumoto K, Date K, Shimura H, Tanaka M, Nakamura T . HGF/NK4, a four-kringle antagonist of hepatocyte growth factor, is an angiogenesis inhibitor that suppresses tumor growth and metastasis in mice. Cancer Res 2000; 60: 6737–6743.
Yue D, Wang Y, Ma P, Li Y-Y, Chen H, Wang P et al. Effects of transferred NK4 gene on proliferation, migration, invasion and apoptosis of human prostate cancer DU145 cells. Asian J Androl 2010; 12: 381–389.
Choi J, Jung W, Koo JS . Expression of autophagy-related markers beclin-1, light chain 3A, light chain 3B and p62 according to the molecular subtype of breast cancer. Histopathology 2013; 62: 275–286.
Bjørkøy G, Lamark T, Johansen T . p62/SQSTM1: a missing link between protein aggregates and the autophagy machinery. Autophagy 2006; 2: 138–139.
Huang WC, Xie Z, Konaka H, Sodek J, Zhau HE, Chung LWK . Human osteocalcin and bone sialoprotein mediating osteomimicry of prostate cancer cells: role of cAMP-dependent protein kinase A signaling pathway. Cancer Res 2005; 65: 2303–2313.
Bendinelli P, Maroni P, Matteucci E, Luzzati A, Perrucchini G, Desiderio MA . Hypoxia inducible factor-1 is activated by transcriptional co-activator with PDZ-binding motif (TAZ) versus WWdomain-containing oxidoreductase (WWOX) in hypoxic microenvironment of bone metastasis from breast cancer. Eur J Cancer 2013; 49: 2608–2618.
Maroni P, Matteucci E, Luzzati A, Perrucchini G, Bendinelli P, Desiderio MA . Nuclear co-localization and functional interaction of COX-2 and HIF-1α characterize bone metastasis of human breast carcinoma. Breast Cancer Res Treat 2011; 129: 433–450.
Whelan KA, Reginato MJ . Surviving without oxygen: hypoxia regulation of mammary morphogenesis and anoikis. Cell Cycle 2011; 10: 2287–2294.
Maes H, Rubio N, Garg AD, Agostinis P . Autophagy: shaping the tumor microenvironment and therapeutic response. Trends Mol Med 2013; 19: 428–446.
Bendinelli P, Matteucci E, Maroni P, Desiderio MA . NF-kB activation, dependent on acetylation/deacetylation, contributes to HIF-1 activity and migration of bone metastatic breast carcinoma cells. Mol Cancer Res 2009; 7: 1328–1341.
Desiderio MA . Hepatocyte growth factor in invasive growth of carcinomas. Cell Mol Life Sci 2007; 64: 1341–1354.
Taddei ML, Giannoni E, Fiaschi T, Chiarugi P . Anoikis: an emerging hallmark in health and diseases. J Pathol 2012; 226: 380–393.
Chen K, Perez-Stable C, D’Ippolito G, Schiller PC, Roos BA, Howard GA . Human bone marrow-derived stem cell proliferation is inhibited by hepatocyte growth factor via increasing the cell cycle inhibitors p53, p21 and p27. Bone 2011; 49: 1194–1204.
Soto AM, Sonnenschein C . The tissue organization field theory of cancer: A testable replacement for the somatic mutation theory. Bioassays 2011; 33: 332–340.
Valastyan S, Weinberg RA . Tumor metastasis: molecular insights and evolving paradigms. Cell 2011; 147: 275–292.
Mizuno S, Nakamura T . HGF-MET cascade, a key target for inhibiting cancer metastasis: the impact of NK4 discovery on cancer biology and therapeutics. Int J Mol Sci 2013; 14: 888–919.
Wei L, Dai Q, Zhou Y, Zou M, Li Z, Lu N et al. Oroxylin A sensitizes non-small cell lung cancer cells to anoikis via glucose-deprivation-like mechanisms: c-Src and hexokinase II. Biochim Biophys Acta 2013; 1830: 3835–3845.
Woolley JF, Corcoran A, Groeger G, Landry WD, Cotter TG . Redox-regulated growth factor survival signaling. Antioxid Redox Signal 2013 doi:10.1089/ars.2012.5028.
Qi X-J, Wildey GM, Howe PH . Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J Biol Chem 2006; 281: 813–823.
Ruppert SM, Li W, Zhang G, Carlson AL, Limaye A, Durum SK et al. The major isoforms of Bim contribute to distinct biological activities that govern the processes of autophagy and apoptosis in interleukin-7 dependent lymphocytes. Biochim Biophys Acta 2012; 1823: 1877–1893.
Giannoni E, Fiaschi T, Ramponi G, Chiarugi P . Redox regulation of anoikis resistance of metastatic prostate cancer cells: key role for Src and EGFR-mediated pro-survival signals. Oncogene 2009; 28: 2074–2086.
Whelan KA, Caldwell SA, Shahriari KS, Jackson SR, Franchetti LD, Johannes GJ et al. Hypoxia suppression of Bim and Bmf blocks anoikis and luminal clearing during mammary morphogenesis. Mol Biol Cell 2010; 21: 3829–3837.
Emaduddin M, Bicknell DC, Bodmer WF, Feller SM . Cell growth, global phosphotyrosine elevation, and c-Met phosphorylation through Src family kinases in colorectal cancer cells. Proc Natl Acad Sci USA 2008; 105: 2358–2362.
Kim JI, Lakshmikanthan V, Frilot N, Daaka Y . Prostaglandin E2 promotes lung cancer cell migration via EP4-betaArrestin1-c-Src signalosome. Mol Cancer Res 2010; 8: 569–577.
Rucci N, Recchia I, Angelucci A, Alamanou M, Del Fattore A, Fortunati D et al. Inhibition of protein kinase c-Src reduces the incidence of breast cancer metastasis and increases survival in mice: implications for therapy. J Pharmacol Exp Ther 2006; 318: 161–172.
Hu YL, Jahangiri A, De Lay M, Aghi MK . Hypoxia-induced tumor cell autophagy mediates resistance to anti-angiogenic therapy. Autophagy 2012; 8: 979–981.
Karar J, Maity A . PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci 2011; 4: 51.
Ponomarev V, Doubrovin M, Serganova I, Vider J, Shavrin A, Beresten T et al. A novel triple-modality reporter gene for whole-body fluorescent, bioluminescent, and nuclear noninvasive imaging. Eur J Nucl Med Mol Imag 2004; 31: 740–751.
Rice L, Lepler S, Pampo C, Siemann DW . Impact of the SRC inhibitor dasatinib on the metastatic phenotype of human prostate cancer cells. Clin Exp Metast 2012; 29: 133–142.
Jie JZ, Wang JW, Qu JG, Wang W, Hung T . Effects of adenoviral-mediated gene transduction of NK4 on proliferation, movement, and invasion of human colonic LS174T cancer cells in vitro. World J Gastroenterol 2006; 12: 3983–3988.
Bretland AJ, Lawry J, Sharrard RM . A study of death by anoikis in cultured epithelial cells. Cell Prolif 2001; 34: 199–210.
Matteucci E, Castoldi R, Desiderio MA . Hepatocyte growth factor induces pro-apoptotic genes in HepG2 hepatoma but not in B16-F1 melanoma cells. J Cell Physiol 2001; 186: 387–396.
Maemondo M, Narumi K, Saijo Y, Usui K, Tahara M, Tazawa R et al. Targeting angiogenesis and HGF function using an adenoviral vector expressing the HGF antagonist NK4 for cancer therapy. Mol Ther 2002; 5: 177–185.
Acknowledgements
This work was supported by CARIPLO Foundation (2010-0737) (MAD and GS), by Ministero della Salute Ricerca Corrente L4022, L4046 and L4069 (PM) and by Università degli Studi di Milano PUR 2009 (PB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by G Raschellá
Supplementary Information accompanies this paper on Cell Death and Disease website
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41419-022-04991-7
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Maroni, P., Bendinelli, P., Matteucci, E. et al. RETRACTED ARTICLE: Osteolytic bone metastasis is hampered by impinging on the interplay among autophagy, anoikis and ossification. Cell Death Dis 5, e1005 (2014). https://doi.org/10.1038/cddis.2013.465
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2013.465
Keywords
This article is cited by
-
Retraction Note to: Epigenetic regulation of HGF/Met receptor axis is critical for the outgrowth of bone metastasis from breast carcinoma
Cell Death & Disease (2022)
-
Retraction Note: HGF and TGFβ1 differently influenced Wwox regulatory function on Twist program for mesenchymal-epithelial transition in bone metastatic versus parental breast carcinoma cells
Molecular Cancer (2020)
-
Dasatinib prevents skeletal metastasis of osteotropic MDA-MB-231 cells in a xenograft mouse model
Archives of Gynecology and Obstetrics (2020)
-
RETRACTED ARTICLE: Epigenetic regulation of HGF/Met receptor axis is critical for the outgrowth of bone metastasis from breast carcinoma
Cell Death & Disease (2017)
-
Deconvoluting the relationships between autophagy and metastasis for potential cancer therapy
Apoptosis (2016)