Abstract
Growth arrest and DNA-damage-inducible, beta (GADD45β) has been reported to inhibit apoptosis via attenuating c-Jun N-terminal kinase (JNK) activation. We demonstrated here that GADD45β mediated its anti-apoptotic effect via promoting p53 protein degradation following arsenite treatment. We found that p53 protein expression was upregulated in GADD45β−/− cells upon arsenite exposure as compared with those in GADD45β+/+ cells. Further studies showed that GADD45β attenuated p53 protein expression through Src/protein phosphatase 2A/murine double minute 2-dependent p53 protein-degradation pathway. Moreover, we identified that GADD45β-mediated p53 protein degradation was crucial for its anti-apoptotic effect due to arsenite exposure, whereas increased JNK activation was not involved in the increased cell apoptotic response in GADD45β−/− cells under same experimental conditions. Collectively, our results demonstrate a novel molecular mechanism responsible for GADD45β protection of arsenite-exposed cells from cell death, which provides insight into our understanding of GADD45β function and a unique compound arsenite as both a cancer therapeutic reagent and an environmental carcinogen. Those novel findings may also enable us to design more effective strategies for utilization of arsenite for the treatment of cancers.
Similar content being viewed by others
Main
Growth arrest and DNA-damage-inducible, beta (GADD45β) is a member of GADD45 family, and is originally cloned as a myeloid-differentiation primary-response gene (also named MyD118).1 GADD45β is a small (18 kD) protein quickly induced by stressful factors, such as ultraviolet (UV) radiation2 and hypoxia.3 It has been reported that TNF-α treatment induces GADD45β protein expression through nuclear factor κB (NFκB)-mediated transcription,4 and transforming growth factor-β induces its expression in Smad-dependent manner.5 In contrast to proapoptotic effect of GADD45α,6, 7 GADD45β has been characterized as an anti-apoptotic protein. For example, GADD45β mediates hepatocyte survival during liver regeneration8 and protects IL-1β-treated INS-1E cells from apoptosis.9 GADD45β-deficient cell is more sensitive to UV-induced apoptosis.2 Subsequent studies indicate that GADD45β can tightly bind to MAPK kinase 7 (MKK7) and attenuate its kinase activity, and in turn results in inactivation of MKK7/c-Jun N-terminal kinase (JNK) apoptotic pathway.10, 11 GADD45β expression synergistically represses cell growth through interaction with PCNA and p21,12, 13 and inhibits cdc2/cyclin B1 kinase activity and in turn induces G2/M arrest.14 GADD45β can also directly bind to MTK1/MEKK4 and enhance those kinase autophosphorylation and activity,15 and subsequently activate downstream kinases, JNK/p38.15, 16 Although anti-apoptotic effect of GADD45β is well-documented in previous studies, role of GADD45β in regulation of tumor-suppressor p53 expression and function has not been explored yet.
Tumor-suppressor p53 is a transcription factor responsible for transcriptional regulation of several key genes implicated in cell cycle control, DNA repair, and apoptosis.17, 18, 19 Although GADD45α is a well-known p53-regulated gene,20 GADD45β is identified as p53-independent gene.2 Because p53 and GADD45β are response genes upon oxidative stress, elucidation of potential cross-talk between those two pathways will be essential for understanding of their biological significance in oxidative stress responses. Our current study found that GADD45β accelerated p53 protein degradation via targeting Src/protein phosphatase 2A (PP2A)/murine double minute 2 (MDM2) pathway.
Results
GADD45β protected cells from death through JNK-independent pathway upon arsenite treatment
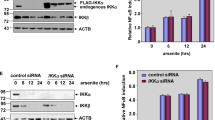
GADD45β has been reported to protect hematopoietic cells from UV-induced apoptosis in JNK-dependent pathway,2 and our previous study shows that arsenite treatment induces GADD45β protein expression.6 To evaluate potential role and molecular basis of GADD45β induction in arsenite response, GADD45β+/+ and GADD45β−/− cells were employed. As shown in Figure 1a, arsenite treatment increased GADD45β protein expression in GADD45β+/+ cells, whereas as expected, there was no detectable GADD45β in GADD45β−/− cells. Our results also showed that arsenite exposure resulted in markedly cell death in a dose-dependent manner in GADD45β−/− cells, whereas GADD45β+/+ cells only showed a slightly morphological alteration and inhibition of cell growth under same experimental conditions (Figure 1b). Consistent with cell morphological alterations, flow-cytometry analysis suggested that arsenite treatment led to significant cell death in GADD45β−/− cells, whereas there was no detectable cell death in GADD45β+/+ cells under the same treatment (Figure 1c). This cell death was due to apoptosis because the cell death was consistent with the results obtained from detection of two well-characterized cell apoptotic markers, cleaved caspase 3 and cleaved poly (ADP-ribose) polymerase (PARP) (Figure 1a). Our results strongly suggested that GADD45β induction by arsenite did exhibit a protection from cell death. As published studies have shown that GADD45β suppressed cell apoptosis through directly binding to MKK7 and inhibiting JNK activation,2, 8, 11 we compared MAPKs activation between GADD45β+/+ and GADD45β−/− cells following arsenite treatment. Consistent with previous reports, GADD45β deficiency (GADD45β−/−) resulted in increased JNK activation by arsenite in comparison with that in GADD45β+/+ cells (Figure 2a), and p38 and extracellular signal-regulated kinase (Erk) activation was also slightly elevated in GADD45β−/− cells (Figure 2a). To determine whether elevation of JNK activation had a role in the increased apoptosis upon arsenite treatment, a specific JNK inhibitor SP600125 was employed. The results showed that inhibition of JNK activation by SP600125 pretreatment did not show observable reduction of arsenite-induced apoptosis indicated by cleaved caspase 3 and cleaved PARP (Figure 2b). These results suggested that although JNK activation was elevated in GADD45β−/− cells, it did not contribute to increased sensitivity of GADD45β−/− cells to arsenite-induced apoptotic responses.
GADD45β protected arsenite-treated cells from death. GADD45β+/+ and GADD45β−/− cells were seeded into six-well plate till 80% confluent. The cell culture medium was replaced with 0.1% FBS DMEM for 12 h and then subjected to arsenite treatment at indicated doses. (a) The cells were extracted, and protein samples were subjected to western blotting with specific antibodies as indicated. (b) Cell morphology images were taken under microscope. (c) Cells were subjected to flow-cytometry analysis as described in Materials and Methods
GADD45β exhibited its protective effect through JNK-independent pathway following arsenite treatment. (a) GADD45β+/+ and GADD45β−/− cells were seeded into six-well plate till 80% confluent. The cell culture medium was replaced with 0.1% FBS DMEM for 12 h and then subjected to arsenite treatment for 3 h as indicated. The cells were extracted, and protein samples were subjected to western blotting with specific antibodies against total and phosphorylated MAPKs. (b) GADD45β−/− cells were seeded into six-well plate. The cells were pretreated with SP600125 for 30 min and were then exposed to arsenite as indicated. Cell extracts were subjected to western blotting with specific antibodies as indicated
GADD45β promoted p53 protein degradation through elevating MDM2 phosphorylation in arsenite responses
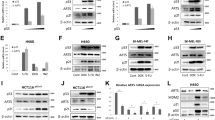
Our most recent study has shown that arsenite-induced p53 protein induction via p50 (NFκB1)-mediated miR-190/PH-domain and leucine-rich repeat protein phosphatase 1/Akt pathway is essential for apoptotic response.21 To test whether GADD45β participated in the regulation of p53 protein expression upon arsenite exposure, we evaluated p53 protein induction in both GADD45β+/+ and GADD45β−/− cells. As shown in Figure 3a, arsenite-induced p53 protein level was remarkably increased in GADD45β−/− cells compared with that in GADD45β+/+ cells. It has been known that p53 phosphorylation at Ser15 attenuated its binding with MDM2 and enhanced p53 protein accumulation.22 Thus, we also determined p53 phosphorylation at Ser15 in both cell lines. The results showed that consistent with total p53 protein expression, p53 phosphorylation at Ser15 was also elevated in GADD45β−/− cells. The results obtained from determination of p53 mRNA levels in both cell lines strongly revealed that p53 mRNA level was regulated by neither arsenite treatment nor GADD45β expression (Figure 3b), suggesting that GADD45β might mediate p53 protein expression at either protein degradation or translation. We therefore compared p53 protein-degradation rates between GADD45β+/+ and GADD45β−/− cells. The data showed that p53 protein-degradation rate in GADD45β−/− cells was much slower than that in GADD45β+/+ cells (Figure 3c), and arsenite treatment could delay p53 protein degradation in both cell lines (Figure 3c). In contrast to p53 protein, p21 protein degradation was faster in GADD45β−/− cells as compared with that in GADD45β+/+ cells (Figure 3c). It has been well known that MDM2 phosphorylation at Ser166 increases its binding activity to p53 protein and mediates p53 protein degradation.22, 23 So we compared MDM2 phosphorylation at Ser166 between the GADD45β+/+ and GADD45β−/− cells following arsenite treatment. The results indicated that MDM2 phosphorylation at Ser166 was much lower in GADD45β−/− cells in comparison with that in GADD45β+/+ cells (Figure 3d), however, GADD45β deletion did not affect total MDM2 expression (Figure 3d), suggesting that GADD45β regulated p53 protein degradation via mediating MDM2 protein phosphorylation at Ser166, rather than affecting total MDM2 expression.
GADD45β depletion stabilized p53 protein through dephosphorylating MDM2. (a) GADD45β+/+ and GADD45β−/− cells were exposed to arsenite as indicated dose for 12 h. The cell extracts were subjected to western blotting with specific antibodies against p-p53 Ser15, p53, GADD45α, Bax, and PUMA. (b) After arsenite treatment for 6 h, total RNA was extracted, and reverse transcription was performed as described in Materials and Methods. Bax, puma, and p53 mRNA level in GADD45β+/+ and GADD45β−/− cells with or without arsenite treatment was analyzed by PCR. (c) GADD45β+/+ and GADD45β−/− cells were seeded into six-well plate and were then pretreated with 10 μM of MG132 for 4 h. The cell culture medium was replaced by fresh medium containing 10 μM CHX with or without arsenite as indicated. The cells were extracted for determination of p53 and p21 protein levels at time points as indicated. (d) GADD45β+/+ and GADD45β−/− cells were treated by 10 μM of arsenite for indicated time, and cell extracts were subjected to western blotting with specific antibodies against p-MDM2 Ser166 or MDM2. (e and f) The cells of HCT116, HCT116/Bax−/− and HCT116/PUMA−/− were identified (e), and the expressions of caspase3 and cleaved caspase3 in these three cells with or without arsenite treatment as indicated dose were detected using western blotting assay (f)
To determine whether the upregulation of p53 protein expression, and p53 phosphorylation at Ser15, in GADD45β−/− cells could mediate p53-regulated gene expression, we also evaluated expression of GADD45α, Bax and PUMA in both GADD45β+/+ and GADD45β−/− cells. The results showed that GADD45α protein expression was markedly increased in GADD45β−/− cells in comparison with that in GADD45β+/+ cells (Figure 3a), whereas there was no observable difference of Bax and PUMA protein expression between GADD45β+/+ and GADD45β−/− cells following arsenite exposure, although arsenite treatment showed an increased PUMA protein expression and no effect on Bax protein expression in both cell lines (Figure 3a). Consistent with their protein expression, the mRNA levels of bax and puma was comparable between GADD45β+/+ and GADD45β−/− cells (Figure 3b). Our previous study has demonstrated that arsenite treatment induces apoptotic response via p50/GADD45α/JNK-dependent mitochondrial pathway.6 Most recently, it has also been reported that arsenite could promoted human neural stem cell apoptosis through mitochondria-dependent signal pathway.24 Although Bax and PUMA are reported to have essential role in mitochondria-dependent cell apoptotic responses,25 Bax-independent apoptotic pathways have also been reported in previous studies. For example: Panton–Valentine leukocidin could directly target to mitochondria and induce Bax-independent cell apoptosis,26 and serum withdrawal-induced apoptosis could also be observed in Bax/Bak double-knockout cells.27 To further exclude the potential involvement of Bax and PUMA in the apoptotic responses by arsenite, the HCT116 Bax−/− and HCT116 PUMA−/− cells were employed. The results indicated that deletion of either Bax or PUMA (Figure 3e) did not show observable reduction of caspase 3 cleavage following arsenite treatment (Figure 3f). Taken together, our results suggested that arsenite-induced apoptosis might be mediated via GADD45α-dependent and Bax- and PUMA-independent mitochondrial pathway.
GADD45β mediated MDM2 phosphorylation at Ser166 via regulation of PP2A phosphorylation at Tyr307
MDM2 phosphorylation at Ser166 is regulated by multiple pathways.23, 28 MEK/Erk activation has been reported to positively regulate MDM2 phosphorylation at Ser166 in HepG2 cells.23 Phophoinositide 3-kinase (PI3K)/Akt also has an important role in modulation of MDM2 phosphorylations at Ser166 and Ser186.28 The results obtained from our comparison of Akt activation did not show any observable difference between GADD45β+/+ and GADD45β−/− cells following arsenite treatment, whereas Erk phosphorylation induced by arsenite was slightly increased in GADD45β−/− cells (Figure 4a). Thus, we anticipated that both Erk and Akt might not participate in GADD45β-regulated MDM2 phosphorylation at Ser166. Phosphotase PP2A is consisted of regulatory subunits A and B, and catalytic subunit C,29 and has been shown to be implicated in dephosphorylation of MDM2 at Ser166.30 C subunit phosphorylation at Tyr307 negatively regulates PP2A catalytic activity.29 Our data revealed that arsenite treatment led to marked increase in PP2A C subunit phosphorylated at Tyr307 in GADD45β+/+ cells, while it was attenuated in GADD45β−/− cells under same experimental conditions (Figure 4b). Moreover, there was no difference on total PP2A C subunit protein levels between two cell lines (Figure 4b). To identify whether PP2A protein binds to MDM2 protein, co-immunoprecipitation was performed to pull down MDM2 protein using specific anti-MDM2 antibody. As shown in Figure 4c, PP2A C subunit was detected in immunocomplex pull down with anti-MDM2 antibody. Importantly, more PP2A protein presented in the immunocomplex showed an inverse correlation with levels of p-MDM2 at Ser166 (Figure 4c), consistently supporting our notion that PP2A is a phosphotase targeting p-MDM2 at Ser166. Moreover, arsenite treatment attenuated PP2A binding to MDM2 in GADD45β+/+ cells, whereas it increased this binding in GADD45β−/− cells (Figure 4c). To detect PP2A function in regulating MDM2 phosphorylation at Ser166 and p53 expression, okadaic acid (OA),31 the inhibitor of PP2A was employed. As shown in Figure 4d, the phosphorylation of MDM2 at Ser166 was markedly increased in GADD45β−/− cells upon OA treatment for 12 h, whereas arsenite-induced p53 protein expression was reduced in same experimental conditions. Our study demonstrated that GADD45β had an important role in downregulation of PP2A interaction and regulation of MDM2 functions following arsenite exposure.
GADD45β-mediated p53 protein degradation via regulating PP2A/MDM2 pathway. (a, b and e) GADD45β+/+ and GADD45β−/− cells were exposed to arsenite, and cell extracts were subjected to western blotting with indicated specific antibodies. (c) Total cell lysate of GADD45β+/+ and GADD45β−/− cells with the indicated treatment for 9 h was subjected to co-immunoprecipitation assay using α-MDM2 antibody. Immunocomplex samples were further subjected to western blotting with specific antibodies against MDM2, p-MDM2 Ser166, and PP2A C subunit. (d) GADD45β−/− cells seeded into six-well plate were pretreated with OA for 30 min and then exposed to arsenite for 12 h. The cell extracts were subjected to western blotting with specific antibodies against p53 and MDM2. (f) Stable transfectants of GADD45β+/+(nonsense) and GADD45β+/+(siSrc) were seeded into six-well plates. When cell confluence reached 70–80%, cell culture medium was replaced with 0.1% FBS DMEM and cultured for 12 h. The cells were then exposed to arsenite for another 12 h. Cell extracts were subjected to western blotting
GADD45β regulated Src phosphorylation following arsenite exposure
It has been found that Src, a non-receptor tyrosine kinase, has a key role in regulation of PP2A C subunit phosphorylation and its function.32, 33 Src kinase activity is positively regulated by its autophosphorylation at Tyr416, whereas it is negatively regulated by phosphorylation at Tyr527.33 To test potential involvement of Src activation in GADD45β regulating PP2A phosphorylation, we compared Src phosphorylation status between GADD45β+/+ and GADD45β−/− cells following arsenite exposure. The results, as expected, showed that in GADD45β+/+ cells, p-Src Tyr416 was remarkably increased after arsenite treatment (Figure 4e), whereas this phosphorylation was dramatically downregulated in GADD45β−/− cells (Figure 4e). In addition, phosphorylated Src at Tyr527 had no dramatic change in GADD45β+/+ cells, but markedly increased in GADD45β−/− cells upon arsenite treatment (Figure 4e). These results revealed that GADD45β depletion resulted in downregulation of Src activity. As Src regulation of PP2A phosphorylation is well characterized in previous studies, we anticipate that increased Src activity in GADD45β+/+ cells might be responsible for reduction of PP2A phosphorylation and activity. Thus, small interfering RNA specific to mouse Src (siSrc) were transfected into GADD45β+/+ cells, and the stable transfectants were identified after puromycin selection. As shown in Figure 4f, Src expression was markedly knockdown in GADD45β+/+ (siSrc) cells in comparison with that in GADD45β+/+ (nonsense) cells. Consistently, the phosphorylation of PP2A at Tyr307 was also dramatically decreased in GADD45β+/+ (siSrc) cells as compared with that in control cells (Figure 4f), whereas the p-MDM2 at Ser166 was attenuated and the p53 and p-p53 at Ser15 were subsequently upregulated in GADD45β+/+ (siSrc) cells upon arsenite treatment (Figure 4f). Taken together, our results demonstrated that PP2A was involved in downregulation of MDM2 phosphorylation at S166, which led to p53 protein accumulation in GADD45β−/− cells possibly through suppressing Src function.
Elevation of p53 expression in GADD45β−/− cells was responsible for increased sensitivity of apoptotic response following arsenite treatment
To test whether p53 upregulation rendered the increased arsenite apoptotic response in GADD45β−/− cells, small interfering RNA targeting mouse p53 was transfected into GADD45β−/− cells, and stable transfectant GADD45β−/− (sip53) and its no-silencing vector control transfectant GADD45β−/− (vector) were established and identified as indicated in Figure 5c. Arsenite-induced cell death was dramatically inhibited in GADD45β−/− (sip53) compared with GADD45β−/− (vector) cells (Figures 5a and b). Cell apoptosis induced by arsenite was dramatically reduced as indicated by cleaved caspase 3 and PARP (Figure 5c). The results strongly demonstrated that p53 protein upregulation in GADD45β−/− cells mediated the increased apoptotic response due to arsenite treatment.
p53 protein accumulation in GADD45β-depletion cells had a role in arsenite-induced cell death. The construct of mouse interfering RNA specific to p53 (sip53) and its empty vector were stably transfected into GADD45β−/− cell. After starvation, the stable transfectants were exposed to arsenite for 12 h. (a) The cell morphology images were taken under microscope. (b) Cells were collected and subjected to flow cytometry for determination of cell death with PI staining. (c) The cells were extracted and subjected to western blotting with indicated specific antibodies
Discussion
p53 is a well-known tumor-suppressor gene, and has an essential role in regulating cell apoptosis upon oxidative stresses.34 Previous study has reported that arsenite induces cell apoptosis in p53-dependent pathway.35 Furthermore, the studies from us and other groups have shown that arsenite treatment fails to upregulate either wild-type or mutant p53 transcription,36, 37 whereas arsenite treatment promotes mutant p53 protein degradation.37 In current study, GADD45β depletion alleviated p53 protein degradation via suppressing MDM2 phosphorylation at Ser166 upon arsenite exposure. MDM2 recognizes and binds to the N-terminal transactivation domain of p53. This binding not only inhibits p53-dependent transcriptional activity and its translocation38 but also functions as an E3 ligase and mediates p53 protein degradation via 26S proteasome.39 p53 is stabilized by phosphorylation at N-terminal residues Ser15 and Ser20, which alleviated its interaction with MDM2.22, 40 MDM2 phosphorylation at Ser395, Ser407 or Thr216 has also been reported to inhibit p53 transfer from nucleus to cytoplasm;30, 31, 41 whereas p-MDM2 at Ser166 enhances its interaction with p53 and promotes p53 protein degradation via MEK/Erk or PI3K/Akt pathway.23, 28 Bax and PUMA, which are implicated in mitochondria-dependent cell apoptosis, are also the important downstream genes of p53.25 PUMA could trigger Bax to translocate into mitochondria and subsequently promote cytochrom-c release, and in turn mediates the mitochondria-dependent cell apoptosis.42, 43 However, Bax-independent apoptotic pathways have also been reported in previous studies.26, 27 It has been reported that Panton–Valentine leukocidin could directly target to mitochondria and induce Bax-independent cell apoptosis,26 and serum withdrawal-induced apoptosis could also be observed in Bax/Bak double-knockout cells.27 In current study, the differential expression of Bax and PUMA in both protein and mRNA levels was not observed between GADD45β+/+ and GADD45β−/− cells following arsenite exposure, and further knockout of either bax or puma did not inhibit arsenite-induced apoptosis. Collectively, we anticipate that GADD45β induction negatively regulated p53 protein accumulation following arsenite treatment through its positive regulation of MDM2 phosphorylation at Ser166, and inhibits cell apoptosis via Bax/PUMA-independent manner.
PP2A is a serine/threonine phosphatase, which consists of two regulatory subunits (A and B) and one catalytic subunit (C).29 It has been reported that PP2A is able to use MDM2 as a substrate and dephosphorylates MDM2 at Ser166.30 Moreover, knockdown of PP2A C subunit increases p-MDM2 at Ser166 and attenuates diosmin-induced p53 protein expression.44 Src is a kinase that mediates PP2A phosphorylation at Tyr307, which inactivates PP2A activity.32 Src consists of a N-terminal SH3 domain, a central SH2 domain and a tyrosine kinase domain.45 Its phosphorylation at Tyr527 by C-terminal Src kinase (CSK) or CSK homology kinase enhances intra-molecularly interaction with SH2 domain, and this interaction conflicts with its autophosphorylation at Tyr416, which activates tyrosine kinase domain.45 Our results showed that GADD45β depletion increased arsenite-induced Src phosphorylation at Tyr527 and decreased its phosphorylation at Tyr416 (Figure 4e), indicating that GADD45β expression provided an inhibitory effect on Src phosphorylation at Tyr527, and further led to elevation of its phosphorylation at Tyr416 and subsequently upregulation of Src kinase activity. It has been reported that GADD45β deletion results in protein kinase A (PKA) activation upon arsenite treatment.46 PKA is an important cyclic adenosine monophosphate47-dependent kinase, and can elevate CSK activation and subsequently enhance p-Src at Tyr527.48 Our results showed that arsenite treatment induced GADD45β protein expression, which might result in PKA inactivation, and in turn further inhibiting Src phosphorylation at Tyr527, and elevating Src phosphorylation at Tyr416, and subsequently upregulating Src kinase activity in GADD45β+/+ cells. The activated Src mediates p-PP2A at Tyr307, and then attenuates PP2A activity. The GADD45β-mediated inactivation of PP2A further results in reduction of its interaction with MDM2 and increased MDM2 phosphorylation at Ser166, and MDM2-mediated p53 protein degradation.
As an important anti-apoptosis gene, GADD45β implicates in regulating cell apoptosis and cancer therapy in many previous studies. NFκB is a key transcription factor responsible for regulation of GADD45β expression.4 Metallopanstimulin-1, the regulator of NFκB activation, inhibits human gastric cancer cell apoptosis by elevating GADD45β expression in NFκB-dependent manner.49 Antitumor drug parthenolide induces breast cancer cell apoptosis by inhibiting NFκB activation and GADD45β expression.47 Another regulator of GADD45β expression is the nuclear receptor constitutive active/androstane receptor (CAR),50 and subsequent study shows that CAR can attenuate TNF-α-induced hepatocellular carcinoma cell apoptosis through forming complex with GADD45β and repressing MKK7/JNK activity.51 GADD45β also protects against INS-1E cell apoptosis upon IL-1β treatment through downregulating JNK and Erk activation.9 In this study, we found that GADD45β provided a protective effect on arsenite-induced cell death in a JNK-independent manner via mediating p53 protein degradation. As a target gene of p53,20 GADD45α is involved in promoting arsenite-induced cell apoptosis via JNK-dependent pathway.6 Our current study showed that GADD45α protein induction was observed accompanied with increased p53 protein accumulation in GADD45β−/− cells upon arsenite treatment (Figure 3a); and knockdown of p53 expression also profoundly attenuated GADD45α protein induction in GADD45β−/− cells (Figure 5c). Thus, our data provide a novel evidence demonstrating a cross-talk between GADD45β and GADD45α upon oxidative stress: with arsenite treatment, activated IKK/NFκB mediates GADD45β induction, and GADD45β induction by arsenite implicates in elevating p53 protein degradation, which represses its target gene GADD45α expression, and further inhibits cell apoptosis, whereas IKK/NFκB1(p50) mediates GADD45α protein accumulation and induces cell apoptosis through activating JNK signal pathway.6 Thus, the balance of GADD45α and GADD45β expression and their cross-talk in their expression regulation has a pivotal role in determination of cell death and survival.
Arsenite is a well-known carcinogen, and long-term exposure to low-dose arsenite ingested from drinking water is associated with high risk of many cancers, including liver, skin, lung, and kidney cancer.52 Our in vitro studies also demonstrate that chronic exposure of cells to low-dose arsenite leads to cell transformation in mouse Cl41, human keratinocyte, and human bronchial epithelial Bease-2B cells.53, 54, 55 Our current study found that GADD45β induction by arsenite mediated an inhibitory effect on p53 protein accumulation, by which it protected cells from death. Because cell death is an important response against oncogenesis by eliminating genetically damaged cells following oxidative stress,56 we anticipate that GADD45β induction by arsenite might be associated with carcinogenic effect of arsenite chronic exposure.
In conclusion, our study demonstrates that GADD45β induction increases p53 protein degradation through inhibiting MDM2 phosphorylation at Ser166 in Src/PP2A-dependent pathway following arsenite treatment. It is the first time, to the best of our knowledge, to demonstrate that GADD45β is implicated in regulating p53 protein degradation, and this novel finding facilitates our understanding of the mechanisms involved in the GADD45β regulation of cell death and provides evidence, indicating that the cross-talk between GADD45α and GADD45β in the regulation of their protein expression is essential for determination of cell death and survival in oxidative stress, such as arsenite exposure.
Materials and Methods
Plasmids, antibodies and other reagents
Mouse small interfering RNA specific to p53 (sip53) and its scramble vector were constructed and reported in our previous studies.57 The constructs of shRNA-targeting mouse Src (siSrc) and its nonsense vector were kind gift from Dr. Gary E. Gallick (Department of Cancer Biology, M.D. Anderson Cancer Center, University of Texas).58 The antibodies specific against p53, p-p53 Ser15, Akt, p-Akt Thr308, phosphatase and tensin homolog, JNK, p-JNK at Thr183/Tyr185, p38, p-p38 at Thr180/Tyr182, Erk, p-Erk Thr202/Tyr204, p-MDM2 Ser166, Src, p-Src Tyr416, N-p-Src Tyr416, p-Src Tyr527, N-p-Src Tyr527, p-PP2A Tyr307, Bax, PUMA, PARP, and caspase 3 were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies specific against GADD45α, GADD45β, MDM2, and p21 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). β-Actin antibody was bought from Sigma (St. Louis, MO, USA). PP2A antibody was purchased from Epitomics (Burlingame, CA, USA). Chemicals of MG132, CHX, and SP600125 were purchased from Calbiochem (San Diego, CA, USA). PP2A inhibitor OA was purchased from LC laboratories (Woburn, MA, USA).
Cell culture and transfectants
GADD45β+/+ and GADD45β−/− mouse embryonic fibroblasts were derived from GADD45β+/+ and GADD45β−/− mice as described,59 and cultured in DMEM medium. The human colon cancer cells HCT116, HCT116/Bax−/− and HCT116/PUMA−/−, as previously described,60 were cultured in McCoy’s 5A medium. All cells and their transfectants were cultured in corresponding medium supplied with 10% FBS, 1% penicillin/streptomycin, and 2 mM L-glutamine (Life Technologies, Grand Island, NY, USA) in 37 °C incubator with 5% CO2. The shRNA-targeting mouse p53 (sip53) or its scramble vector was transfected into GADD45β−/− cells with pSUPER-LacZ, and stable transfectants were selected using hygromycin (Cellgro, Manassas, VA, USA). SiSrc or its vector together with pSUPER-puro was used to transfect GADD45β+/+ cells. The stable transfectants were selected by puromycin (Cellgro). And all transfectants were mediated by PolyJet DNA in Vitro Transfection Reagent (SignaGen Laboratories, Rockville, MD, USA).
Reverse transcription PCR
Cells treated by arsenite with indicated doses for 6 h were subjected to total RNA extraction using TRIzol reagent (Invitrogen, Grand Island, NY, USA) following the manufacturer’s instructions. Total RNA (5 μg) was used for first-strand cDNA synthesis with oligdT(20) primer by SuperScript III First-Strand Synthesis system (Invitrogen). Specific primers (Invitogen) for mouse p53 (forward: 5′-CAC GTA CTC TCC TCC CCT CA-3′, reverse: 5′-CTT CTG TAC GGC GGT CTC TC-3′), bax (forward: 5′-TCG AGC AGG GAG GAT GGC TG-3′, reverse: 5′-TTC CCA GCC ACC CTG GTC TT-3′), puma (forward: 5′-CTC AGC CCT CCC TGT CAC CA-3′, reverse: 5′-CGC CGC TCG TAC TGC GCG TT-3′), and β-actin (forward: 5′-CCT GTG GCA TCC ATG AAA CT-3′, reverse: 5′-GTG CTA GGA GCC AGA GCA GT-3′) were used for PCR amplification.
Protein-degradation assay
GADD45β+/+ and GADD45β−/− cells were seeded into six-well plate, respectively. When cell confluence reached 70–80%, MG132 (10 μM) was used to pretreat cells for 4 h, and then the medium was replaced with fresh medium containing 10 μM CHX with or without 10 μM arsenite and cells were cultured for indicated time periods. The cells were extracted and subjected to western blotting for determination of p53 protein levels.
Immunoprecipitation
To determine PP2A interaction with MDM2, GADD45β+/+ and GADD45β−/− cells were cultured in 10-cm dishes till 70–80% confluence. Then, cell culture medium was replaced with 0.1% FBS DMEM and cultured for 12 h. Cells were then treated with arsenite for 9 h. Cell lysate was collected with IP buffer (25 mM Tris-HCl, pH7.5, 1 mM DTT, 30 mM MgCl2, 40 mM NaCl, 0.5% NP-40, and protease inhibitor). Total protein (1 mg) from each sample was subjected to immunoprecipitation using antibodies specific against MDM2 (anti-MDM2; Sigma) or control mouse IgG together with protein A/G plus agarose (Santa Cruz Biotechnology). Agarose were collected after centrifugation (5000 × g, 2 min) at 4 °C and washed using IP buffer for 4–5 times. SDS sample buffer (2 × ) was used to extract the proteins from agarose beads for western blotting.
Flow cytometry
Cells were seeded into six-well plates and cultured until 70–80% confluence. The cell culture medium was replaced with 0.1% FBS DMEM and cultured for another 12 h, and the cells were then exposed to arsenite as indicated. All cells were collected by centrifugation at 1500 r.p.m. for 5 min. The cell pellets were washed by ice-cold PBS, following by fixing in ice-cold 70% ethanol at −20 °C overnight. Then, cells were washed with PBS 2–3 times and cell death was analyzed using flow cytometry (Beckman, Indianapolis IN, USA) after incubating with PI buffer (0.1% Triton X-100, 0.2 mg/ml RNase A, and 0.05 mg/ml PI) for 15 min.
Western blotting
Cells were seeded into six-well plates and cultured until 70–80% confluent. The cell culture medium was replaced with 0.1% FBS medium for 12 h and then subjected to arsenite treatment as indicated time period. The cells were extracted with cell lysis buffer (10 mM Tris-HCl, pH 7.4, 1% SDS, 1 mM Na3VO4, and proteasome inhibitor), and protein concentration was determined by Nano Drop 2000 (Thermo Scientific, Holtsville, NY, USA). Protein extract (30–60 μg/sample) was subjected to SDS-PAGE gel, and western blotting was carried out as described in our previous report.6
Abbreviations
- NFκB:
-
Nuclear factor κB
- JNK:
-
c-Jun N-terminal kinase
- Erk:
-
extracellular signal-regulated kinase
- MKK:
-
MAPK kinase
- PP2A:
-
protein phosphatase 2A
- GADD45β:
-
growth arrest and DNA-damage-inducible beta
- PI3K:
-
phophoinositide 3-kinase
- MDM:
-
murine double minute
- PARP:
-
poly (ADP-ribose) polymerase
- OA:
-
okadaic acid
- UV:
-
ultraviolet
- CSK:
-
C-terminal Src kinase
- PKA:
-
protein kinase A
- CAR:
-
constitutive active/androstane receptor
References
Hoffman B, Liebermann DA . Role of gadd45 in myeloid cells in response to hematopoietic stress. Blood Cells Mol Dis 2007; 39: 344–347.
Gupta M, Gupta SK, Hoffman B, Liebermann DA . Gadd45a and Gadd45b protect hematopoietic cells from UV-induced apoptosis via distinct signaling pathways, including p38 activation and JNK inhibition. J Biol Chem 2006; 281: 17552–17558.
Kim MY, Seo EJ, Lee DH, Kim EJ, Kim HS, Cho HY et al. Gadd45beta is a novel mediator of cardiomyocyte apoptosis induced by ischaemia/hypoxia. Cardiovasc Res 2010; 87: 119–126.
Karin M, Lin A . NF-kappaB at the crossroads of life and death. Nat Immunol 2002; 3: 221–227.
Yoo J, Ghiassi M, Jirmanova L, Balliet AG, Hoffman B, Fornace AJ Jr. et al. Transforming growth factor-beta-induced apoptosis is mediated by Smad-dependent expression of GADD45b through p38 activation. J Biol Chem 2003; 278: 43001–43007.
Song L, Li J, Zhang D, Liu ZG, Ye J, Zhan Q et al. IKKbeta programs to turn on the GADD45alpha-MKK4-JNK apoptotic cascade specifically via p50 NF-kappaB in arsenite response. J Cell Biol 2006; 175: 607–617.
Hildesheim J, Bulavin DV, Anver MR, Alvord WG, Hollander MC, Vardanian L et al. Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Cancer Res 2002; 62: 7305–7315.
Papa S, Zazzeroni F, Fu YX, Bubici C, Alvarez K, Dean K et al. Gadd45beta promotes hepatocyte survival during liver regeneration in mice by modulating JNK signaling. J Clin Invest 2008; 118: 1911–1923.
Larsen CM, Dossing MG, Papa S, Franzoso G, Billestrup N, Mandrup-Poulsen T . Growth arrest- and DNA-damage-inducible 45beta gene inhibits c-Jun N-terminal kinase and extracellular signal-regulated kinase and decreases IL-1beta-induced apoptosis in insulin-producing INS-1E cells. Diabetologia 2006; 49: 980–989.
Papa S, Monti SM, Vitale RM, Bubici C, Jayawardena S, Alvarez K et al. Insights into the structural basis of the GADD45beta-mediated inactivation of the JNK kinase, MKK7/JNKK2. J Biol Chem 2007; 282: 19029–19041.
Papa S, Zazzeroni F, Bubici C, Jayawardena S, Alvarez K, Matsuda S et al. Gadd45 beta mediates the NF-kappa B suppression of JNK signalling by targeting MKK7/JNKK2. Nat Cell Biol 2004; 6: 146–153.
Zhan Q, Lord KA, Alamo I Jr., Hollander MC, Carrier F, Ron D et al. The gadd and MyD genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell growth. Mol Cell Biol 1994; 14: 2361–2371.
Vairapandi M, Balliet AG, Fornace AJ Jr., Hoffman B, Liebermann DA . The differentiation primary response gene MyD118, related to GADD45, encodes for a nuclear protein which interacts with PCNA and p21WAF1/CIP1. Oncogene 1996; 12: 2579–2594.
Vairapandi M, Balliet AG, Hoffman B, Liebermann DA . GADD45b and GADD45g are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stress. J Cell Physiol 2002; 192: 327–338.
Takekawa M, Saito H . A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell 1998; 95: 521–530.
Cho HJ, Park SM, Hwang EM, Baek KE, Kim IK, Nam IK et al. Gadd45b mediates Fas-induced apoptosis by enhancing the interaction between p38 and retinoblastoma tumor suppressor. J Biol Chem 2010; 285: 25500–25505.
Fridman JS, Lowe SW . Control of apoptosis by p53. Oncogene 2003; 22: 9030–9040.
Yin Y, Tainsky MA, Bischoff FZ, Strong LC, Wahl GM . Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell 1992; 70: 937–948.
Teodoro JG, Parker AE, Zhu X, Green MR . p53-mediated inhibition of angiogenesis through up-regulation of a collagen prolyl hydroxylase. Science 2006; 313: 968–971.
Zhan Q . Gadd45a, a p53- and BRCA1-regulated stress protein, in cellular response to DNA damage. Mutat Res 2005; 569: 133–143.
Yu Y, Zhang D, Huang H, Li J, Zhang M, Wan Y et al. NF-κB1 p50 promotes p53 protein translation through miR-190 downregulation of PHLPP1. Oncogene 2013 e-pub ahead print 11 February 2013; doi:10.1038/onc2013.8.
Shieh SY, Ikeda M, Taya Y, Prives C . DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 1997; 91: 325–334.
Malmlof M, Roudier E, Hogberg J, Stenius U . MEK-ERK-mediated phosphorylation of Mdm2 at Ser-166 in hepatocytes. Mdm2 is activated in response to inhibited Akt signaling. J Biol Chem 2007; 282: 2288–2296.
Ivanov VN, Hei TK . Induction of apoptotic death and retardation of neuronal differentiation of human neural stem cells by sodium arsenite treatment. Exp Cell Res 2013; 319: 875–887.
Sinha S, Malonia SK, Mittal SP, Singh K, Kadreppa S, Kamat R et al. Coordinated regulation of p53 apoptotic targets BAX and PUMA by SMAR1 through an identical MAR element. EMBO J 2010; 29: 830–842.
Genestier AL, Michallet MC, Prevost G, Bellot G, Chalabreysse L, Peyrol S et al. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J Clin Invest 2005; 115: 3117–3127.
Zamorano S, Rojas-Rivera D, Lisbona F, Parra V, Court FA, Villegas R et al. A BAX/BAK and cyclophilin D-independent intrinsic apoptosis pathway. PLoS One 2012; 7: e37782.
Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC . HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol 2001; 3: 973–982.
Liu W, Akhand AA, Takeda K, Kawamoto Y, Itoigawa M, Kato M et al. Protein phosphatase 2A-linked and -unlinked caspase-dependent pathways for downregulation of Akt kinase triggered by 4-hydroxynonenal. Cell Death Differ 2003; 10: 772–781.
Okamoto K, Li H, Jensen MR, Zhang T, Taya Y, Thorgeirsson SS et al. Cyclin G recruits PP2A to dephosphorylate Mdm2. Mol Cell 2002; 9: 761–771.
Maya R, Balass M, Kim ST, Shkedy D, Leal JF, Shifman O et al. ATM-dependent phosphorylation of Mdm2 on serine 395: role in p53 activation by DNA damage. Genes Dev 2001; 15: 1067–1077.
Chen J, Parsons S, Brautigan DL . Tyrosine phosphorylation of protein phosphatase 2A in response to growth stimulation and v-src transformation of fibroblasts. J Biol Chem 1994; 269: 7957–7962.
Nho RS, Peterson M . Eukaryotic translation initiation factor 4E binding protein 1 (4EBP-1) function is suppressed by Src and protein phosphatase 2A (PP2A) on extracellular matrix. J Biol Chem 2011; 286: 31953–31965.
Azizi B, Ziaei A, Fuchsluger T, Schmedt T, Chen Y, Jurkunas UV . p53-regulated increase in oxidative-stress--induced apoptosis in Fuchs endothelial corneal dystrophy: a native tissue model. Invest Ophthalmol Vis Sci 2011; 52: 9291–9297.
Keim A, Rossler OG, Rothhaar TL, Thiel G . Arsenite-induced apoptosis of human neuroblastoma cells requires p53 but occurs independently of c-Jun. Neuroscience 2012; 206: 25–38.
Huang C, Ma WY, Li J, Dong Z . Arsenic induces apoptosis through a c-Jun NH2-terminal kinase-dependent, p53-independent pathway. Cancer Res 1999; 59: 3053–3058.
Yan W, Zhang Y, Zhang J, Liu S, Cho SJ, Chen X . Mutant p53 protein is targeted by arsenic for degradation and plays a role in arsenic-mediated growth suppression. J Biol Chem 2011; 286: 17478–17486.
Mayo LD, Donner DB . A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA 2001; 98: 11598–11603.
Kubbutat MHG, Jones SN, Vousden KH . Regulation of p53 stability by Mdm2. Nature 1997; 387: 299–303.
Shieh SY, Taya Y, Prives C . DNA damage-inducible phosphorylation of p53 at N-terminal sites including a novel site, Ser20, requires tetramerization. EMBO J 1999; 18: 1815–1823.
Shinozaki T, Nota A, Taya Y, Okamoto K . Functional role of Mdm2 phosphorylation by ATR in attenuation of p53 nuclear export. Oncogene 2003; 22: 8870–8880.
Zhang Y, Xing D, Liu L . PUMA promotes Bax translocation by both directly interacting with Bax and by competitive binding to Bcl-X L during UV-induced apoptosis. Mol Biol Cell 2009; 20: 3077–3087.
Polster BM, Robertson CL, Bucci CJ, Suzuki M, Fiskum G . Postnatal brain development and neural cell differentiation modulate mitochondrial Bax and BH3 peptide-induced cytochrome c release. Cell Death Differ 2003; 10: 365–370.
Dung TD, Day CH, Binh TV, Lin CH, Hsu HH, Su CC et al. PP2A mediates diosmin p53 activation to block HA22T cell proliferation and tumor growth in xenografted nude mice through PI3K-Akt-MDM2 signaling suppression. Food Chem Toxicol 2012; 50: 1802–1810.
Roskoski R Jr . Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun 2005; 331: 1–14.
Shibuya EK . G2 cell cycle arrest--a direct link between PKA and Cdc25C. Cell Cycle 2003; 2: 39–41.
Sweeney CJ, Mehrotra S, Sadaria MR, Kumar S, Shortle NH, Roman Y et al. The sesquiterpene lactone parthenolide in combination with docetaxel reduces metastasis and improves survival in a xenograft model of breast cancer. Mol Cancer Ther 2005; 4: 1004–1012.
Jin H, Garmy-Susini B, Avraamides CJ, Stoletov K, Klemke RL, Varner JA . A PKA-Csk-pp60Src signaling pathway regulates the switch between endothelial cell invasion and cell-cell adhesion during vascular sprouting. Blood 2012; 116: 5773–5783.
Yang ZY, Qu Y, Zhang Q, Wei M, Liu CX, Chen XH et al. Knockdown of metallopanstimulin-1 inhibits NF-kappaB signaling at different levels: the role of apoptosis induction of gastric cancer cells. Int J Cancer 2012; 130: 2761–2770.
Yamamoto Y, Negishi M . The antiapoptotic factor growth arrest and DNA-damage-inducible 45 beta regulates the nuclear receptor constitutive active/androstane receptor-mediated transcription. Drug Metab Dispos 2008; 36: 1189–1193.
Yamamoto Y, Moore R, Flavell RA, Lu B, Negishi M . Nuclear receptor CAR represses TNFalpha-induced cell death by interacting with the anti-apoptotic GADD45B. PLoS ONE 2010; 5: e10121.
Chen CJ, Chen CW, Wu MM, Kuo TL . Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br J Cancer 1992; 66: 888–892.
Ouyang W, Luo W, Zhang D, Jian J, Ma Q, Li J et al. PI-3K/Akt pathway-dependent cyclin D1 expression is responsible for arsenite-induced human keratinocyte transformation. Environ Health Perspect 2008; 116: 1–6.
Zhang D, Li J, Gao J, Huang C . c-Jun/AP-1 pathway-mediated cyclin D1 expression participates in low dose arsenite-induced transformation in mouse epidermal JB6 Cl41 cells. Toxicol Appl Pharmacol 2009; 235: 18–24.
Carpenter RL, Jiang Y, Jing Y, He J, Rojanasakul Y, Liu LZ et al. Arsenite induces cell transformation by reactive oxygen species, AKT, ERK1/2, and p70S6K1. Biochem Biophys Res Commun 2011; 414: 533–538.
Zhuang L, Wang B, Sauder DN . Review: molecular mechanism of ultraviolet-induced keratinocyte apoptosis. J Interferon Cytokine Res 2000; 20: 445–454.
Wang J, Ouyang W, Li J, Wei L, Ma Q, Zhang Z et al. Loss of tumor suppressor p53 decreases PTEN expression and enhances signaling pathways leading to activation of activator protein 1 and nuclear factor kappaB induced by UV radiation. Cancer Res 2005; 65: 6601–6611.
Trevino JG, Summy JM, Lesslie DP, Parikh NU, Hong DS, Lee FY et al. Inhibition of SRC expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am J Pathol 2006; 168: 962–972.
Ju S, Zhu Y, Liu L, Dai S, Li C, Chen E et al. Gadd45b and Gadd45g are important for anti-tumor immune responses. Eur J Immunol 2009; 39: 3010–3018.
Luo W, Liu J, Li J, Zhang D, Liu M, Addo JK et al. Anti-cancer effects of JKA97 are associated with its induction of cell apoptosis via a Bax-dependent and p53-independent pathway. J Biol Chem 2008; 283: 8624–8633.
Acknowledgements
This work was partially supported by grants from NIH/NCI CA112557, NIH/NIEHS ES000260, NSF81229002, NSF9102970, and NBRPC 2012CB525004.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by D Aberdam
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Yu, Y., Huang, H., Li, J. et al. GADD45β mediates p53 protein degradation via Src/PP2A/MDM2 pathway upon arsenite treatment. Cell Death Dis 4, e637 (2013). https://doi.org/10.1038/cddis.2013.162
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2013.162
Keywords
This article is cited by
-
Andrographolide induces vascular smooth muscle cell apoptosis through a SHP-1-PP2A-p38MAPK-p53 cascade
Scientific Reports (2014)