Abstract
The molecules that mediate death of selective neurons in Alzheimer’s disease (AD) are mostly unknown. The Forkhead transcription factor FoxO3a has emerged as an important mediator of cell fate including apoptosis. When phosphorylated by Akt, it is localized in the cytosol as an inactive complex bound with 14-3-3 protein. For activation and localization of FoxO3a in the nucleus, further modifications are required, such as phosphorylation by mammalian sterile 20-like kinase 1 (MST1) and arginine methylation by protein arginine methyltransferase1. We report here that Akt-mediated phosphorylation of FoxO3a is diminished in neurons exposed to oligomeric β-amyloid (Aβ), in vitro and in vivo. We also find that oligomeric Aβ activates FoxO3a by MST1 phosphorylation and arginine methylation in primary cultures of hippocampal and cortical neurons. Moreover, FoxO3a translocates from the cytosol to nucleus in cultured neurons in response to Aβ. Most importantly, the nuclear redistribution of FoxO3a is significantly increased in Aβ-overexpressing AβPPswe-PS1dE9 mice and Aβ-infused rat brains. We further find that FoxO3a is essential for loss of neurons and neural networks in response to Aβ. Recent reports implicate Bim, a pro-apoptotic member of Bcl-2 family, in neuron death in AD, as a key target of this transcription factor. We show that Bim is a direct target of FoxO3a in Aβ-treated neurons. Our findings thus indicate that FoxO3a is activated, translocated to the nucleus and mediates neuron death via Bim in response to Aβ toxicity.
Similar content being viewed by others
Main
Alzheimer’s disease (AD) is the most common progressive neurodegenerative disease characterized by synapse and neuron loss, formation of neurofibrillary tangles and senile plaques in selective areas of the brain.1, 2 A central hypothesis in AD pathogenesis is that the accumulation and oligomerization of β-amyloid (Aβ) peptide due to altered proteolytic processing of amyloid precursor protein and/or clearance of this peptide is an early and critical event leading to widespread synaptic dysfunction and selective neuronal loss.3 In addition to genetic evidence that Aβ accumulation triggers neurodegeneration in vivo, in vitro studies reveal that oligomeric Aβ robustly induces neuronal apoptosis.3, 4, 5, 6 Transcriptional activation of death-associated genes is required for apoptosis.7 However, the identity of transcription factors and the mechanisms by which these factors induce neuronal apoptosis in AD are incompletely understood.
FoxO3a, a Forkead transcription factor of Forkhead box, class ‘O’ (FoxO) subfamily, has been implicated in a variety of death paradigms, including neuronal apoptosis.8 In mammals, four subclasses of FoxO have been identified. These are FoxO1/FKHR,9 FoxO3a/FKHRL-1,10, 11 FoxO4/AFX12, 13, 14 and FoxO6.15 The transcriptional activity of FoxO3a is modulated by its various post-translational modifications.16, 17 Among them, phosphorylation is the most important one. Phosphorylation of FoxO3a at different sites by different kinases may have completely different consequences. If it is phosphorylated in response to growth factor or insulin stimulation by survival kinases such as Akt, it is excluded out from the nucleus,18, 19 and degraded through ubiquitination by the E3 ubiquitin ligase Skp2.20 On the other hand, FoxO3a is activated through phosphorylation by several stress kinases. For example, if it is phosphorylated at Ser207 by mammalian sterile 20-like kinase 1 (MST1) in response to oxidative stress, it accumulates in the nucleus and causes cell death.21, 22 FoxO proteins are also activated by methylation. Recently, Yamagata et al.23 have shown that methylation of FoxO1 at Arg248 and Arg250 of mouse by protein arginine methyltransferase1 (PRMT1) in response to oxidative stress results in enhanced nuclear localization of this protein. This methylation actually abrogates Akt-induced phosphorylation of FoxO1 at Ser253 and thereby inhibits its nuclear exclusion and subsequent proteosomal degradation.
Activated FoxO3a can upregulate multiple genes depending on the apoptotic stimuli. Among them, Bim is of particular interest, as it is already implicated in AD.4, 24 Bim is one of the BH3-only members of Bcl-2 family whose proapoptotic function is critical for a number of neuron death paradigms.25 It has been reported that in response to NGF deprivation, Akt-mediated phosphorylation of FoxO3a is attenuated, which results in its nuclear localization and induction of Bim.8, 26 Bim gene has two consensus sequences for FoxO protein binding, and it has been shown that FoxO3a specifically binds with bim in response to NGF deprivation.8
It is conceived by researchers that there is no simple solution for a complex disease like AD, and a combinatorial treatment may provide effective measures for this disease. Because of the definitive role of impaired Aβ metabolism, a number of drugs targeted at Aβ metabolism are in clinical trials to reduce the Aβ load. However, most of them are discontinued due to lack of effect or toxicity. Even if a drug lowers the Aβ load, a complementary therapy is required to make the brain more resistant to Aβ, which cannot be removed effectively and safely.1 Therefore, deeper understanding of the mechanism of Aβ-induced death is essential. In this study, we investigated whether FoxO3a has any role in Aβ-induced neuron death and our findings suggest that FoxO3a is activated by multiple post-translational modifications, translocates to the nucleus, binds with Bim and induces neuron death in response to Aβ toxicity.
Results
Activation of FoxO3a by multiple post-translational modifications in response to Aβ1-42
As PI3K/Akt signalling is reported to be markedly inhibited in AD,27 we investigated whether phosphorylation at the Akt site of FoxO3a is reduced in a cellular model of AD. For this, we used a primary culture of rat cortical neurons and treated them with oligomeric Aβ1-42 or Aβ42-1. The Aβ1-42 induced significant neuron death after 24 h at 1.25 μM concentration. In contrast, the reverse peptide Aβ42-1 had no effect on neuronal survival even at 4 μM concentration (Supplementary Figure S1). A time course study revealed that phosphorylation of FoxO3a at Thr32, which is one of the sites phosphorylated by Akt, is reduced at 4 h and greatly diminished after 16 h of Aβ1-42 exposure (Figure 1a and b). To determine whether this decline in the phospho-FoxO3a level was due to the decline in the total level of FoxO3a, we checked the total FoxO3a level, and found no significant reduction in the total FoxO3a level even after 16 h of Aβ1-42 treatment (Figures 1a and b). The ratio of FoxO3a/pFoxO3a(Thr32) increased significantly with time following Aβ1-42 exposure (Figure 1c). However, Aβ42-1 did not alter the ratio of FoxO3a/pFoxO3a (Supplementary Figure S2), indicating an Aβ1-42-specific increase in the level of active form of FoxO3a.
Level of phosphorylation at Thr32 of FoxO3a is drastically reduced in response to Aβ. (a) Primary cortical neurons were exposed to oligomeric Aβ1-42 (1.5 μM) for indicated times. The total cell lysates were prepared as described in the experimental procedure and then proteins were separated by SDS-PAGE and probed by western blotting using enhanced chemiluminescence for the level of total FoxO3a and phosphorylated FoxO3a by using specific antibodies against FoxO3a and p-FoxO3a (Thr32). A representative immunoblot showing FoxO3a and p-FoxO3a levels is depicted. Actin level was used as loading control. (b) Graphical representation of the level of FoxO3a and p-FoxO3a as determined by scanning and quantification with NIH imageJ program. Data represent mean±S.E.M. of six independent experiments. *P<0.05. (c) Graphical representation of the ratio of total FoxO3a and p-FoxO3a protein level in cortical neurons exposed to Aβ. This ratio represents the level of active form of FoxO3a protein in the cell. Data are represented as mean±S.E.M. of six independent experiments. Asterisks denote statistically significant differences between 0 h (control) and 4, 8, 16 h of Aβ treatment: *P<0.001
Next, we examined whether FoxO3a is phosphorylated by MST1 as well in response to Aβ1-42. Primary cultures of rat cortical neurons were treated with Aβ1-42 and phosphorylation of FoxO3a at ser207 was checked by a phospho-Ser207-specific antibody. A time course study revealed that phosphorylation of FoxO3a at Ser207 is significantly increased within 4 h and is peaked at 8 h of Aβ1–42 exposure (Figures 2a and b). The ratio of pFoxO3a(Ser207)/FoxO3a increased with time after Aβ1-42 treatment (Figure 2c). An immunocytochemical study also showed that phosphorylated FoxO3a at Ser207 is localized in the nucleus of majority of cells after 8 h of Aβ1-42 treatment (Figure 2d).
MST1-mediated phosphorylation of FoxO3a at Ser207 and arginine methylation of FoxO3a increase on Aβ exposure. (a) Primary cortical neurons were exposed to oligomeric Aβ1-42 (1.5 μM) for the indicated times. The total cell lysates were separated by SDS-PAGE and subjected to western blotting using enhanced chemiluminescence. Specific antibody was used to detect the level of FoxO3a phosphorylated at serine 207. A representative immunoblot showing p-FoxO3a(S207) levels is depicted. Actin level was used as loading control. (b) Graphical representation of densitometric results of p-FoxO3a(S207) levels as determined by NIH imageJ program. Data are represented as mean±S.E.M. of three independent experiments. Asterisks denote statistically significant differences between 0 h (control) and 4, 8, 16 h of Aβ treatment: *P<0.01. (c) Graphical representation of ratio of PhosphoFoxO3a (S207) and total FoxO3a protein level in cortical neurons exposed to Aβ. This ratio represents the level of active form of FoxO3a protein in the cell. Data represented as mean±S.E.M. of three independent experiments. Asterisks denote statistically significant differences between 0 h (control) and 4, 8, 16 h of Aβ treatment: *P<0.001. (d) Differentiated PC12 cells were treated with Aβ1-42 (5 μM) for 8 h and immunostained with pFoxO3a(S207) antibody. Alexa Fluor 568 was used as secondary antibody. The nucleus was stained with Hoechst. Fluorescence microscope images show an increase in the level of pFoxO3a(S207) in cells exposed to Aβ1-42 (5 μM). (e) Methylation of FoxO3a at arginine residues in response to Aβ. Primary rat cortical neurons were treated with oligomeric Aβ1-42 (1.5 μM) for 8 h. The cell lysates were analysed for the level of FoxO3a methylated at the arginine residues. For this, total FoxO3a was immunoprecipitated using anti-FoxO3a antibody followed by western blotting by anti-dimethyl arginine antibody. A representative immunoblot showing increase in the arginine methylation of FoxO3a in Aβ1-42-treated cells is depicted. (f) Graphical representation of densitometric analysis of levels of FoxO3a methylated at arginine residues as determined by NIH imageJ program. Data are represented as mean±S.E.M. of three independent experiments. Asterisks denote statistically significant differences between control and treatment: *P<0.01. (g) Primary rat cortical neurons were treated with or without Aβ1-42 in the presence and absence of a chemical inhibitor of PRMT1. Quantitative data show significant protection of cells by the chemical inhibitor of PRMT1 against Aβ1-42-induced death. Data are represented as mean±S.E.M. of three independent experiments.*P<0.05
The arginine residues of FoxO1 that are methylated by PRMT1 are conserved in FoxO3a. We tested whether arginine methylation of FoxO3a occurs in neurons exposed to Aβ1-42. Primary cultures of rat cortical neurons were treated with Aβ1-42 for 8 h, then cells were lysed and immunoprecipitated with FoxO3a antibody and western blotted with dimethyl arginine antibody. Results showed increased arginine methylation of FoxO3a in neurons treated with Aβ1-42 compared with control (Figures 2e and f). The results were confirmed by immunoprecipitation followed by western blot in the opposite direction, that is, immunoprecipitation with dimethyl arginine antibody and western blotting with FoxO3a (Supplementary Figure S3). Moreover, a chemical inhibitor of PRMT1 significantly protected cortical neurons from Aβ1-42-induced death (Figure 2g). Taken together, these findings suggest that FoxO3a is activated in neurons in response to Aβ1-42 treatment by multiple mechanisms that may collectively lead to its nuclear localization.
Activation of FoxO3a in vivo in response to Aβ1-42 infusion
We have also examined the activation of FoxO3a in response to Aβ1-42 in vivo. Adult rats were infused with oligomeric Aβ1-42 in the brain, as described by Frautschy et al.28 They have shown that injection of Aβ into rat cortex and hippocampus resulted in neuronal loss and Aβ deposition in the vicinity of injection sites. After 21 days, brain sections were immunostained with anti-Aβ1-42 and caspase3 antibodies to validate the model. The results showed the presence of Aβ deposition and an elevated level of active caspase3 in neurons in the region of injection (Figures 3a–c). Haematoxylin and eosin staining of brain sections showed loss of neuronal cells in hippocampus near the infusion site of Aβ1-42. Neuronal loss was absent when animals were infused with reverse peptide, Aβ42-1 (Supplementary Figure S4). Aβ deposition and elevated level of active caspase3 were not detected in PBS-infused rat brains (Figure 3c). Most importantly, phosphorylation of FoxO3a at the Akt site Thr32 was greatly reduced and the nuclear distribution of FoxO3a was greatly enhanced in the neurons in Aβ1-42-infused brains, but not in PBS- or Aβ42-1-infused brains (Figures 3d and data not shown). Thus it is clear that FoxO3a is activated in response to Aβ1-42 in vivo as well.
FoxO3a is dephosphorylated following Aβ1-42 infusion in vivo. (a) The rats were infused with either Aβ1-42 or PBS in the right hemisphere of the brain in the treated or control group, respectively. After 21 days of infusion, the animals were killed and the brains were taken out following cardiac perfusion. The frozen brains were sectioned by cryotome. Representative image of a brain section stained with haematoxylin–eosin is shown. (b) Brain sections of the treated rats were immunostained with Aβ1-42 antibody to check the presence of Aβ plaques in the infused area. Upper panel shows brain section stained with anti-Aβ1-42 antibody. Lower panel shows brain sections from the same animal stained without any primary antibody as a negative control. (c) Brain sections (portion marked with black box in a) of control and treated groups were co-immunostained for active caspase3 and NeuN. Nuclei were stained with Hoechst. Arrows show neurons with high active caspase-3 staining. Representative image of six sections from three animals with similar results is shown here. Images were taken under × 40 objective. (d) Brain sections (portion marked with black box in a) of control and treated groups were co-immunostained for p-FoxO3a(Thr32) and NeuN. Nuclei were stained with Hoechst. Representative images of six sections from three animals with similar result are shown here. Scale bar, 50 μm. (e) Confocal images of brain sections from control and treated groups were co-immunostained for FoxO3a and NeuN. Nuclei were stained with Hoechst. Representative images of six sections from three animals with similar results are shown here. Scale bar, 10 μm. (f) Enlarged view of the first column of d, showing cells from treated and control brains immunostained with p-FoxO3a(Th32). Arrows show neurons with high or low p-FoxO3a(Th32) staining. (g) Enlarged view of the first column of e, showing cells from treated and control brains immunostained with FoxO3a. Arrows show the neurons with cytosolic or nuclear FoxO3a
FoxO3a translocates from cytosol to nucleus in neurons in response to Aβ1-42
Next, we checked activation-associated nuclear translocation of FoxO3a by determining its subcellular localization in neurons exposed to Aβ1-42. Western blotting analysis of cytosolic and nuclear fractions revealed a progressive reduction of total FoxO3a in the cytosol with a corresponding increase in the nucleus following Aβ1-42 exposure, and almost complete translocation in the nucleus after 16 h of Aβ treatment (Figures 4a and b). The cross-contamination of nuclear and cytosolic fractions was checked by using actin and methyl histone antibody, which are cytosolic and nuclear markers, respectively (Figure 4a and Supplementary Figure S5). These results corroborate well with the timing of dephosphorylation of the protein at Akt site, phosphorylation at Ser207 by MST1 and upregulation of its target gene, Bim.4
FoxO3a is translocated from the cytosol to the nucleus in neuronal cells in response to Aβ. (a and b) Primary cortical neurons (7DIV) were exposed to oligomeric Aβ1-42 (1.5 μM) for the indicated times. The cytosolic and nuclear extracts were isolated as described in the experimental procedure and proteins were analysed by western blotting, as described in Figure 1a, for the level of FoxO3a. (a) Representative immunoblots showing change in FoxO3a levels in the cytosol (left) and nucleus (right). (b) Graphical representation of change in FoxO3a protein level in the cytosol (left) and nucleus (right). Asterisks denote statistically significant differences between 0 h (control) and 4, 8, 16 h of Aβ treatment: *P<0.05. (c) Aβ exposure causes the translocation of FoxO3a from the cytosol to the nucleus in differentiated PC12 cells. Differentiated PC12 cells (5DIV) were treated with Aβ for 16 h. Cells were then fixed and immunostained with FoxO3a antibody and Hoechst. Localization of FoxO3a was visualized by using fluorescence microscopy. (d) Graphical representation of FoxO3a translocation in differentiated PC12 cells following Aβ exposure as quantified by NIH imageJ. Data represent mean±S.E.M of seven different cells from two independent experiments. Asterisks denote statistically significant differences between control and treatment: *P<0.001. (e) Aβ exposure causes the translocation of FoxO3a from the cytosol to the nucleus in hippocampal neurons. Hippocampal neurons (24DIV) were maintained with or without Aβ for 16 h. Cells were then fixed and immunostained with FoxO3a. The nucleus was stained with Hoechst. Localization of FoxO3a was visualized by using confocal microscopy
To further confirm this Aβ-induced nuclear translocation of FoxO3a, we performed immunostaining of cytoplasmic and nuclear FoxO3a in neuronally differentiated PC12 cells and in cultures of rat hippocampal neurons following overnight exposure to Aβ1-42. Fluorescence and confocal microscopy revealed that FoxO3a immunostaining is present mostly in the cytosol in control cells. A significant increase in FoxO3a staining occurs in the nucleus with drastic decrease in the cytosol of cells exposed to Aβ1-42 (Figure 4c). Furthermore, quantification of FoxO3a in the nucleus and cytosol of confocal images of immunostained PC12 cells by using NIH-imageJ revealed that the nuclear to cytosolic ratio of FoxO3a fluorescence intensity was significantly increased in Aβ1-42-treated cells compared with control cells (Figure 4d). As in the case of neuronal PC12 cells, Aβ1-42 exposure to cultures of rat hippocampal neurons also enhanced nuclear localization of FoxO3a in hippocampal neurons with minimal retention of the protein in cytosol (Figure 4e). Taken together, these results indicate a clear and significant translocation of FoxO3a into the nucleus in neuronal cells exposed to Aβ1-42.
Nuclear distribution of FoxO3a in neurons enhanced in AβPPswe-PS1dE9 mice
As we used a synthetic Aβ in our study, which could have different effects than naturally secreted Aβ, we examined the nuclear redistribution of FoxO3a in Aβ-overexpressing AβPPswe-PS1dE9 mice.29 Examination of sections from adult transgenic or wild-type littermates confirmed the formation of Aβ plaques in transgenic mice (Figure 5a). Immunohistochemical studies also revealed that FoxO3a was present in the nucleus of most of the cortical neurons in transgenic animals, but not in the control littermates (Figures 5b and c). Moreover, quantification of FoxO3a by using NIH-imageJ revealed that the nuclear to cytosolic ratio of FoxO3a fluorescence intensity was significantly high in transgenic animals compared with the control animals (Figure 5d). These results corroborated our experiments with synthetic Aβ1-42 peptide.
Increased nuclear distribution of FoxO3a in neurons of AβPPswe-PS1dE9 transgenic mice. The brain tissues from AβPPswe-PS1dE9 transgenic mice and control littermate were analysed for FoxO3a subcellular distribution. (a) Congo red staining of brain slices from transgenic and wild-type mice. Transgenic mice brain shows the presence of amyloid plaques (shown by arrow), whereas such plaques were absent in the wild-type brain tissue. Representative images of nine sections from three animals of each group with similar results are shown here. Images taken under × 5 objective. (b) Brain sections of wild-type and transgenic animals were co-immunostained for FoxO3a and NeuN. Nuclei were stained with Hoechst. Representative images of five sections from three animals of each group with similar results are shown here. Scale bar, 50 μm. (c) Enlarged view of the first three columns of B showing cells from transgenic and wild-type brains immunostained with FoxO3a. Scale bar, 10 μm. (d) Graphical representation of distribution of FoxO3a in the cytosol and nucleus in cortical neurons of wild-type or transgenic animals as quantified by NIH imageJ. Data represent mean±S.E.M. of about 60 different neurons from three animals of each group. Asterisks denote statistically significant differences between wild-type and transgenic animals: *P<0.001
FoxO has a required role in neuron death evoked by Aβ1-42
We next investigated whether FoxO3a is required for death of neurons in a cellular model of AD. We used different cell types and a previously described short hairpin RNA (shRNA) construct against FoxO genes.26 Knockdown of FoxO significantly protected cortical neurons from death evoked by Aβ1-42 exposure (Figures 6a and b). Most of the shFoxO-expressing neurons (80% viability) survived compared with an irrelevant shRNA (shRand)-expressing neurons (50% viability) (Figure 6b) with good preservation of neuron morphology after 24 h of Aβ1-42 treatment (Figure 6a).
siRNA-mediated knockdown of FoxO and FoxO3a protects the primary rat cortical, hippocampal neurons against Aβ toxicity. (a) Primary rat cortical neurons (7DIV) were transfected with shRand-zsgreen or shFoxO-zsgreen. Forty-eight hours post transfection, oligomeric Aβ1-42 was treated for 24 h. Live green cells were counted after 24 h under fluorescense microscope. Representative pictures of primary rat cortical neurons that were transfected with shRand-zsgreen or shFoxO-zsgreen and exposed to Aβ. (b) Graphical representation of percentage of the viable cells. Asterisks denote statistically significant differences between shRand and shFoxO: *P<0.01. (c) Primary hippocampal neurons (22 DIV) were transfected with shRand-zsgreen or shFoxO-zsgreen. 48 h post transfection, oligomeric Aβ1-42 was treated for 48 h. Live green cells were counted at indicated times under fluorescense microscope. Representative pictures of primary rat hippocampal neurons that were transfected with shRand-zsgreen or shFoxO-zsgreen and exposed to Aβ for 48 h. (d) Graphical representation of percentage of viable cells. Asterisks denote statistically significant differences from shRand (control): *P<0.01. (e) Primary rat cortical neurons (7DIV) were transfected with shRand-zsgreen or shFoxO3a-zsgreen. Forty-eight hours post transfection, oligomeric Aβ1-42 was treated for 48 h. Live green cells were counted after 24 and 48 h under a fluorescence microscope. Representative images of primary rat cortical neurons that were transfected with shRand-zsgreen or shFoxO3a-zsgreen and exposed to Aβ. (f) Graphical representation of percentage of viable cells. Asterisks denote statistically significant differences from shRand (control): *P<0.01. (g) Downregulation of FoxO protects neuronal processes and networks against Aβ toxicity. Hippocampal neurons transfected with either shRand or shFoxO were treated with Aβ for 48 h and then were imaged under a fluorescence microscope. Sholl analyses of imaged neurons were done as described in the experimental procedure. Data represent mean±S.E.M. of six different neurons from three independent cultures for each class. Asterisks denote statistically significant differences from shRand (control): *P<0.001
A similar experiment with primary hippocampal neurons revealed that FoxO is also required for the death of these neurons in response to Aβ1–42 treatment (Figures 6c and d). shFoxO-expressing hippocampal neurons retained all processes even after 2 days of Aβ1-42 exposure (Figure 6c). Moreover, the shFoxO-expressing neuronal PC12 cells retained their differentiated morphology even after 72 h of Aβ1-42 exposure (Supplementary Figure S6).
From these experiments, it was not clear whether FoxO3a actually mediates death, because the shRNA used in these experiments was directed against all FoxO isoforms. Moreover, we found that FoxO1 was also dephosphorylated and translocated to the nucleus in response to Aβ1-42 (Supplementary Figure S7). However, it has been reported that FoxO3a controls FoxO1 expression.30 Therefore, to check the contribution of FoxO3a in Aβ1-42-induced neuron death, we prepared a shRNA construct that targets specifically FoxO3a (efficacy is shown in Supplementary Figure S8), and we found that shFoxO3a-expressing neurons are protected as good as shFoxO-expressing cells (Figures 6e and f).
It has been reported that different caspases, namely caspase3 and caspase6, are required for cell body apoptosis and degeneration of neurites, respectively.31 Thus we examined quantitatively the loss of neuronal networks similar to neuron loss after downregulation of FoxO in response to Aβ treatment by Sholl analysis.32 Sholl analysis is a quantitative method to characterize the morphological features of an imaged cell. For this purpose, single hippocampal neurons transfected with shRand or shFoxO were analysed by NIH-ImageJ, as described in the experimental procedures. Results showed that the number of crossing remained almost the same before and after the treatment of Aβ1-42 in case of shFoxO-transfected neurons, whereas, there was a significant reduction in the number of crossing in the shRand-transfected neurons (Figure 6g). Taken together, these results suggest that knockdown of FoxO not only protects the neuronal cell body but also the neuronal networks against Aβ toxicity.
FoxO mediates neuron death via Bim in response to Aβ1-42 exposure
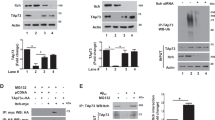
We have shown that Bim is required for the death of cortical neurons evoked by Aβ.4 Gilley et al.8 have shown that FoxO3a directly activates bim gene expression in response to NGF deprivation. We, therefore, were interested to see whether FoxO3a directly activates Bim expression during neuron death evoked by Aβ. First, we checked whether Bim is also upregulated in vivo in response to Aβ, and found that Bim levels were enhanced in cortical neurons after Aβ1-42 infusion in rats (Figure 7a). Moreover, downregulation of Bim by shRNA protected neural networks from Aβ1–42-induced disruption (Supplementary Figure S9). Then we used a previously reported33 luciferase reporter driven by Bim promoter that contains a FoxO-binding site (Figure 7b). As expected, Bim promoter-driven luciferase activity was increased approximately twofold in neuronal PC12 cells that were transiently transfected with this reporter and treated with Aβ1-42 for 24 h (Figure 7c). However, this increase in luciferase activity in response to Aβ1–42 is diminished in cells transfected with shFoxO but not in cells transfected with shRand (Figure 7c). Thus, our result suggests that Bim promoter is activated in response to Aβ and FoxO is necessary for this activation.
FoxO is required for induction of Bim following Aβ exposure. (a) Brain sections of Aβ or PBS-infused rats were co-immunostained for Bim and NeuN. Nuclei were stained with Hoechst. A representative image of six sections from three animals with similar results is shown here. Images were taken under × 20 objective. (b) Schematic representation of Bim-LUC reporter consisting 2.9 kb of the rat bim gene extending 5′ from exon 1. (c) FoxO is necessary for the activation of a Bim promoter-driven luciferase activity in response to Aβ. Neuronally, differentiated PC12 cells were cotransfected with 0.3 μg of Bim-LUC and 0.1 μg of the renilla luciferase expression construct pRL-CMV with 0.4 μg of either shRand or shFoxO per well of 24-well plate. Forty-eight hours post transfection, cells were treated with Aβ for 24 h and processed for luciferase assay. The data are shown as relative firefly luciferase expression normalized to renilla luciferase activity and represent mean±S.E.M. of three independent experiments performed in duplicate/triplicate. (d) shRNAs targeted to FoxO or FoxO3a blocks Bim expression in primary cortical neurons subjected to Aβ exposure. It shows neurons that were transfected with the indicated constructs, maintained for 48 h and then subjected to Aβ for 20 h, after which they were immunostained with antibodies against Bim. Arrows indicate transfected neurons. (e) PRMT1 inhibiton blocks Aβ-induced upregulation of Bim. Cortical neurons were treated with or without Aβ in the presence or absence of PRMT1 inhibitor. The cell lysates were analysed by western blotting for the Bim level. (f) Binding of FoxO3a with endogenous Bim promoter is increased in cultured cortical neurons following Aβ exposure. Primary cultures of rat cortical neurons were treated with or without Aβ for 6 h. An equal number of cells was processed for chromatin immunoprecipitation assay using anti-FoxO3a antibody for immunoprecipitation. The immunoprecipitated materials were subjected to PCR using primers against the portion of Bim promoter that flanks one FoxO-binding site. PCR products were verified by agarose gel electrophoresis. Templates were DNA from cells before chromatin immunoprecipitation (input), DNA in immunoprecipitated (IP) materials with or without FoxO3a antibody or an irrelevant antibody (−ve control). The figure shows a representative image of two independent experiments with similar results
To further confirm our results, we examined the effect of shRNA-mediated knockdown of FoxO on endogenous Bim protein expression in cells exposed to Aβ1-42. Cortical neurons were transfected with shRand, shFoxO or shFoxO3a, and then exposed to Aβ1-42 followed by immunocytochemistry for Bim expression. Results showed that shRNA-mediated knockdown of FoxO or FoxO3a reduced Bim expression substantially in majority of the transfected cells compared with the neighbouring non-transfected cells after Aβ1-42 exposure (Figure 7d).
As FoxO3a is activated by arginine methylation with PRMT1, we determined the role of PRMT1 on Bim expression upon Aβ1-42 exposure by using an inhibitor of PRMT1, and found that its inhibition blocks the Bim induction by Aβ1-42 (Figure 7e).
Next, we performed chromatin immunoprecipitation assay to determine whether FoxO3a specifically binds with Bim promoter in response to Aβ. Results showed that a significant amount of FoxO3a was bound to the endogenous Bim promoter after 6 h of Aβ1-42 exposure. In contrast, there was negligible or no binding in control cells (Figure 7f). An irrelevant antibody did not precipitate Bim DNA, and PCR of FoxO3a immunoprecipitate with primers specific for GAPDH did not produce any product. This is consistent with our previous report4 that showed an elevated Bim mRNA level by this time in rat cortical neurons exposed to Aβ. Collectively, these results suggest that active FoxO3a executes cell death in response to Aβ at least by part, through the upregulation of pro-apoptotic Bim.
Discussion
In this study, we investigated whether FoxO3a is activated and has a necessary role in neurodegeneration evoked by Aβ. A number of our experimental observations suggest that FoxO3a gets activated and executes neuron death in response to Aβ toxicity. First, there are significant reductions in phospho-FoxO3a(Thr32) in vitro and in vivo after Aβ treatment. Thr32 of FoxO3a is specifically phosphorylated by Akt including two other specific sites,34 and this phosphorylation results in its association with 14-3-3 protein and cytosolic localization,35 which lead to proteosomal degradation of this protein.16, 17, 36. Second, FoxO3a is phosphorylated by MST1 at Ser207 in a similar time-dependent manner by which it is dephosphorylated by Akt. It has been previously reported that MST1 mediates neuronal cell death by phosphorylating FoxOs in response to oxidative stress or survival factor deprivation.22, 37 Third, there is a robust increase in the level of asymmetrical methylation of arginine residues of FoxO3a in Aβ-exposed neurons. It has been shown that methylation of FoxO1 by PRMT1 abrogates Akt-induced phosphorylation and subsequent nuclear exclusion of this protein after oxidative stress.23 Fourth, subcellular fractionation followed by western blot and immunocytochemical staining confirms the nuclear localization of FoxO3a in Aβ-treated cells. Moreover, phospho-FoxO3a(Ser207) is shown to be localized in the nucleus of neurons. Importantly, FoxO3a is also accumulated in the nucleus of the neurons in AD transgenic mice and Aβ-infused rat brains. Fifth, RNAi of FoxO3a protects the neurons significantly for long hours and retains the overall neuronal morphology against Aβ toxicity. Finally, FoxO3a is required for Bim induction in response to Aβ. We have shown before that Bim is upregulated in the entorhinal cortex of AD brain and is required for the neuron death evoked by Aβ.4
Accumulating evidence strongly suggest that multiple post-translational modifications are required for nuclear localization of FoxO proteins in response to cellular stress.35 We found that FoxO3a is dephosphorylated at the Akt site, phosphorylated by stress kinase MST-1 and methylated at arginine residues in the same time window viz 8 h after Aβ treatment. We have also shown that FoxO3a is maximally translocated to the nucleus by that time. It seems that these modifications simultaneously help translocate FoxO3a into the nucleus. Initially, dephosphorylation at Akt sites and MST1 phopsphorylation promote nuclear translocation of FoxO3a by disrupting association of FoxOs and protein 14-3-3. In the nucleus, FoxO3a can be dephosphorylated by PP2A, both at Akt and MST-1 sites, and Akt-mediated re-phosphorylation could exclude FoxO3a from the nucleus. However, pre-existing arginine methylation blocks the phosphorylation of this protein by Akt and subsequent exclusion from the nucleus.23 Although our study demonstrates a possible mechanism of FoxO3a activation, it does not exclude the participation of other players that modulate FoxO activity. For example, FoxOs are also phosphorylated by c-Jun N-terminal kinase38 and p38 kinase,39 both of which are activated by Aβ.40 However, it is also reported that MST1 can regulate both c-Jun N-terminal kinase and p38 pathways.41
Recent reports suggest that inhibition of FoxOs might provide neuroprotection in AD. Qin et al.42 have shown that calorie restriction attenuates AD-type neuropathology in AD transgenic mice by inactivating FoxO3a. Calorie restriction induces SIRT1 deacetylase, which renders survival of critical cell types by inhibiting FoxO3a’s ability to induce cell death.43, 44 It has also been shown that insulin signalling protects neuronal cells from Aβ toxicity by inhibiting FoxO3a activity.45 Conversely, Stohr et al.46 have reported that exclusively decreased insulin receptor expression reduces AD-associated mortality independent of FoxO activity. However, it has been shown that Aβ-induced neurological phenotypes in Drosophila are strongly suppressed by dFoxO deficiency.47 An antidepressant drug, fluoxetine, significantly delayed Aβ-induced paralysis in the Caenorhabditis elegans model of Aβ toxicity by reducing DAF-16/FoxO activity.48 Resveratrol, a natural compound with antioxidant properties, which is in clinical trial for AD, has been shown to provide neuroprotective effect by inactivating FoxOs.49 Consistent with these findings, our study has revealed that RNAi of FoxO blocks neuronal loss, including loss of neuronal networks against Aβ toxicity.
A number of pro-apoptotic genes are regulated by FoxOs, including FasL, Bim and PUMA.8, 50, 51 Of interest, Bim is reported to be elevated in AD brain and is required for neuron death in response to Aβ.4 Bim has two FoxO-binding sites, which are occupied by FoxO3a in response to NGF deprivation.8 However, it was not known whether FoxO3a also regulates Bim in response to Aβ. Our study shows that Bim is a target for FoxO3a in Aβ-treated neurons as well. Contributions of other FoxO targets in this death paradigm are under investigation. In contrast, FoxOs can resist oxidative stress by inducing several antioxidant enzymes such as catalase and manganese superoxide dismutase.52 Interestingly, Smith et al.53 have reported that Aβ inactivates FoxO3a by inducing its phosphorylation and neuronal cell death appears to be due to downregulation of its target genes MnSOD and catalase. In that study, the cells were treated with Aβ after 16 h of serum deprivation, and the FoxO3a phosphorylation induced by Aβ was diminished after 40 min. Therefore, it seems that FoxO3a was dephosphorylated at Akt sites in serum-deprived condition and was transiently phosphorylated by Aβ. However, our study shows that FoxO3a is phosphorylated at Akt site in the absence of Aβ, and is dephosphorylated and translocates into the nucleus after 4 h or more of Aβ treatment.
In summary, our findings support a model (Figure 8) in which FoxO3a is phosphorylated by Akt, and remains in the cytosol in the absence of Aβ. On Aβ exposure, Akt-mediated phosphorylation of FoxO3a decreases with simultaneous increase in its phosphorylation at ser207 by MST1 and arginine methylation by PRMT1. These post-translational modifications result in an increased nuclear accumulation of FoxO3a. The nuclear FoxO3a binds directly to the Bim promoter leading to its increased expression, which in turn activates the intrinsic apoptotic pathway resulting in neuron death.
Materials and Methods
Materials
Human recombinant NGF, insulin, progesterone, putrescine, selenium, transferrin and poly-D-lysin were purchased from Sigma (St. Louis, MO, USA). Anti-FoxO3a, anti-p-FoxO3a (Th32), anti-actin, anti-methyl histone antibodies were from Cell Signalling Technology (Denver, MA, USA), anti-Bim antibody was from Abcam (Cambridge, UK) and anti-di-methyl arginine antibody was from Millipore (Billerica, MA, USA). Protein A agarose and HRP-conjugated secondary antibodies were from Santa Cruz Biotechnologies (Dallas, Texas, USA). Lipofectamine 2000, anti-pFoxO3a(S207), Alexa Fluor 488, Alexa Fluor 568 and all the culture mediums were purchased from Invitrogen (Life technologies, Grand Island, NY, USA). Brain tissues of AβPPswe-PS1dE9 mice and control littermates were kindly gifted by Dr. Anant B Patel.29
Preparation of shRNA
FoxO3a-specific (shRNA) was prepared in the pSIREN vector by using the BD knockout RNAi system, according to the manufacturer’s instructions (BD Biosciences, San Jose, CA, USA), on the basis of the following sequence of FoxO3a: 5′-CAACCTGTCACTGCACAGC-3′.
Cell culture
Rat pheochromocytoma cells (PC12) cells were cultured as described previously54 in RPMI medium supplemented with 10% heat-inactivated horse serum (HS) and 5% heat-inactivated fetal bovine serum. Neuronal differentiation was induced by NGF (100 ng/ml) in medium containing 1% horse serum for 6 days before the treatment, as previously described.26 Primary rat neurons were cultured as described previously.6, 55 Briefly, for rat cortical neuron culture, cells from neocortex of E18 day rat were used. The cells were plated on poly-D-lysin -coated culture plates and maintained in DMEM/F12 medium supplemented with insulin (25 μg/ml), glucose (6 mg/ml), transferin (100 μg/ml), progesterone (20 ng/ml), putrescine (60 μg/ml) and selenium (30 ng/ml) for 6 days before treatment. Primary hippocampal neurons were cultured from E18 rat hippocampus. The cells were plated on poly-D-lysin-coated plates and cultured in neurobasal medium supplemented with B27 for 24 days before the treatment.
Preparation of amyloid
HPLC-purified Aβ1-42 was purchased from American Peptide (Sunnyvale, CA, USA) and oligomeric Aβ1-42 was prepared as described previously.56 Briefly, lyophilized Aβ1-42 was reconstituted in 100% 1,1,1,3,3,3 hexafluoro-2-propanol (HFIP) to 1 mM, HFIP was removed by evaporation in a Speed Vac (Eppendorf, Hamburg, Germany), then resuspended in 5 mM anhydrous DMSO. This stock was then stored at −80 °C. The stock was diluted with PBS to a final concentration of 400 μM, and SDS was added to a final concentration of 0.2%. The resulting solution was incubated at 37 °C for 18–24 h. The preparation was diluted again with PBS to a final concentration of 100 μM and incubated at 37 °C for 18–24 h before use.
Cell viability assay
The cell viability was checked by the intact nuclear counting method. This assay was performed as described previously.57 In brief, a detergent containing the buffer was added to the cells that dissolve only the cell membrane, leaving the nuclear membrane intact. The intact nuclei were then counted on a haemocytometer. The number of live cells was expressed as percentage of the total cell population.
Western blotting
Cells were lysed and proteins were analysed by western blotting, as described previously.58 Briefly 50 μg of protein of each condition was resolved in 7.5% SDS-PAGE. Proteins were transferred onto PVDF membrane (Hybond: GE Healthcare, Buckinghamshire, UK) and probed with the respective primary antibodies overnight at 4 °C on a shaker. HRP-conjugated secondary antibodies against the primary antibodies were used. Detection was done by Amersham ECL western blotting detection reagent, according to the manufacturer’s protocol. Bands were detected on an X-ray film (Kodak, Windsor, CO, USA).
Immunocytochemical staining
The neuronal cells were immunostained as described previously.26, 33 In brief, the cells were fixed with 4% PFA for 10 min. After three washes with PBS, the cells were blocked in 3% goat serum in PBS containing 0.1% Triton-X 100 for 2 h. The cells were immunolabelled with anti-FoxO3a antibody in a blocking solution overnight at 4 °C. Alexa Fluor 488 was used as secondary antibody and the nucleus was stained with Hoechst.
Chromatin immunoprecipitation
Chromatin immunoprecipitation assays were done by using a CHIP assay kit from Millipore (Billerica, MA, USA) with few exceptions as mentioned. 5–8 × 106 cortical neurons were used after treatment with or without Aβ. Rabbit polyclonal anti-FoxO3a antibody was used to immuneprecipitate the protein–DNA complexes. The primers used for PCR amplification of the rat Bim promoter were 5′-TAAGTTCCGCTCTGAGAGGT-3′ and 5′-CAGGCTGCGACAGGTAGTG-3′. PCR products were analysed on a 1.5% agarose gel and visualized by staining with ethidium bromide.
Subcellular fractionation
Cortical neurons were treated with Aβ for different time intervals and subjected to subcellular fractionation following the proceduredescribed earlier59 with few modification. The cells were pelletted and resuspended in buffer A (20 mM HEPES, 40 mM KCl, 1 mM DTT, 0.2 mM NaVO4, 2 mM MgCl2, 10% glycerol, 0.1 mM EDTA, 0.1 mM EGTA, 0.25 M sucrose and protease inhibitor). The cells were homogenized with 20–30 strokes. The lysates were then centrifuged for 10 min at 1000 r.p.m. The supernatant, which contained the cytosolic proteins, was carefully stored. The pellet contained the nucleus and the unbroken cells. This pellet was then resuspended in buffer B (0.5% ethylhexadecyldimethylammonium bromide, 0.28% glacial acetic acid, 0.5% Triton-X 100, 2 mM MgCl2, 2.2 mM NaCl, 0.1 × PBS), which lysed the cell membrane but kept the nuclear membrane intact.57 This lysate in buffer B was then centrifuged for 10 min at 2000 r.p.m. The supernatant was discarded and the pellet was washed twice with ice-cold PBS. The resulting pellet, containing the nuclear proteins, was resuspended in buffer C (10 mM HEPES, 500 mM NaCl, 1% Triton-X, 10% glycerol, 1 mM NaF and 1 mM NaVO4). Actin and methyl histone were used, respectively, as the cytosolic and nuclear marker proteins to ensure the validity of our protocol.
Transfection
DNA was prepared with Plasmid Maxi kit (Qiagen, Waltham, MA, USA). For the survival assay, neuronal cells were transfected with 0.5 μg of either pSIREN-FoxO-shRNA-ZsGreen or pSIREN-Rand-shRNA-ZsGreen. For the reporter assay, cells were cotransfected with 0.3 μg of bim-luc reporter, 0.1 μg of renilla vector and 0.3 μg of either pSIREN-FoxO-shRNA-ZsGreen or pSIREN-Rand-shRNA-ZsGreen. Transfections were done in 500 μl of serum-free medium per well of a 24-well plate using lipofectamine 2000. Six hours later, lipofectamine-containing medium was replaced by a fresh complete medium. Cortical and hippocampal neurons transfection was performed on the third and twenty-second days of culture, respectively. Neuronal PC12 cells were transfected on the third day of differentiation.
Luciferase assay
Differentiated PC12 cells were transfected with the constructs as mentioned above. Forty-eight hours post transfection, the cells were treated with or without Aβ1-42 for 24 h. The cells were then washed with PBS and lysed in the buffer provided in Promega luciferase system (Promega, Madison, WI, USA). Dual luciferase assay was done according to the manufacturer’s protocol using Luminometer (Perkin Elmer, Waltham, MA, USA). Relative luciferase activities were obtained by normalizing the firefly luciferase activity against renilla luciferase activity. Data are represented as mean±S.E.M. of three independent experiments performed in triplicate.
Immunoprecipitation
The cortical neurons (6DIV) were treated with or without Aβ1-42 for 8 h. The cells were washed with PBS and lysed in lysis buffer. For immunoprecipitation, agarose-conjugated anti-FoxO3a antibody was prepared by incubating 3 μg anti-FoxO3a antibody with 25 μl of protein A agarose beads for 2 h in 4 °C under shaking condition. These agarose-conjugated FoxO3a antibodies were pelletted down and incubated with treated and untreated cell lysates containing equal amounts of protein overnight at 4 °C under shaking condition. The antigen–antibody complexes were isolated and dissociated by boiling in sample buffer for 4–5 min. The agarose beads were pelletted down and the supernatant was subjected to western blotting, as described previously using anti-di-methyl arginine as primary antibody.
Sholl analysis
To analyse the neuritic branching and complexities, sholl analysis was performed using NIH-ImageJ software, as previously described by Cuesto et al.,32 except for few modifications as mentioned below. At first, matured hippocampal neurons (21DIV) were transfected with shFoxO or shRand as described previously. The green transfected neurons were imaged under a fluorescence microscope. Sholl analysis was performed on low-magnification pictures of single neurons imaged at 0 and 48 h of Aβ1-42 treatment using NIH-ImageJ software (Sholl analysis plugin). A number of concentric cycles were drawn from the cell body with gradually increasing radius of 40 μm in length. Two-dimensional analysis was done to count the number of branches that intersect the successive concentric circle. Data are represented as mean±S.E.M. of six neurons from three independent experiments.
Survival assay
Primary cortical neurons (4DIV), hippocampal neurons (21DIV) and neuronally differentiated PC12 cells were transfected with shRand and shFoxO. Forty-eight hours post transfection, the cells were treated with or without Aβ1-42. The number of surviving transfected neurons (green) were counted just after the treatment and at indicated time intervals, as described previously.26 Data are represented as mean±S.E.M. of three independent experiments performed in triplicate.
Imaging
Transfected neurons were imaged under a fluorescence microscope (Leica, Wetzlar, Germany). The translocation and subcellular localization of FoxO3a were studied on immunostained cells imaged under a fluorescence microscope and spinning disc confocal microscope (Andor, Belfast, UK). The z-stack and 3D animation were done by using a spinning disc confocal microscope (Andor).
Study of translocation
PC12 cells were treated with or without Aβ1-42 overnight. The cells were immunostained as described above, and imaged under a spinning disc confocal microscope. The intensities of FoxO3a in the nucleus and whole cell were quantified separately by NIH-ImageJ software. The amount of FoxO3a in the cytosol was calculated by subtracting the amount of nuclear FoxO3a from the total amount. The ratio of nuclear FoxO3a to cytosolic FoxO3a in treated cells compared with control cells has been used to indicate the translocation.
Oligomeric Aβ infusion in animals
Male Sprague-Dawley rats (300–380 g) were anesthetized by injecting a mixture of xylazine–ketamine and placing them on a steriotaxic frame. Injection was done by using a 27-gauge Hamilton syringe. A volume of 5 μl of 100 μM Aβ in PBS was infused in the right cerebral cortex at stereotaxic co-ordinates from bregma: AP: −4.1, L: 2.5, DV: 1.3 mm, according to the previous report28 and also the rat brain atlas. An equal volume of PBS was injected in control animals. Animals were killed 21 days after injection. The brains were dissected out, following cardiac perfusion, and fixed in 4% PFA for 24 h. They were then incubated in a 30% sucrose solution for another 24 h before proceeding for cryo sectioning. Cryo sections of the brain were done in cryotome (Thermo, West Palm Beach, FL, USA).
Immnohistochemistry of brain slices
Twenty-micrometre cryo sections of the brain from Aβ-infused or PBS-infused rats and wild-type or transgenic mice were blocked with 5% goat serum in PBS containing 0.3% Triton-X 100 for 1 h at room temperature. Brain slices were incubated in primary antibody in a blocking solution overnight at 4 °C. Sections were washed thrice with PBS and incubated with a fluorescence-tagged secondary antibody for 2 h at room temperature. Following three washes with PBS and Hoechst staining for the nucleus, the sections were mounted and observed under fluorescence and confocal microscopes.
Statistics
The experimental results were reported as mean±S.E.M. Student’s t-test was performed as unpaired, two-tailed sets of arrays to evaluate the significance of difference between the means and was presented as P-values. One-way ANOVA was performed for data sets of more than two groups.
Abbreviations
- FoxO:
-
forkhead box, class O
- dFoxO:
-
Drosophila FoxO
- DAF16:
-
dauer formation-16
- Aβ:
-
β-amyloid
- AD:
-
Alzheimer’s disease
- PC12:
-
pheochromocytoma cells
- MST1:
-
mammalian sterile 20-like kinase 1
- PRMT1:
-
protein arginine methyltransferase1
- Bcl-2:
-
B-cell lymphoma 2
- BH:
-
Bcl-2 homology domain
- Bim:
-
Bcl-2-interacting mediator of cell death
- PUMA:
-
p53-upregulated modulator of apoptosis
- RNAi:
-
RNA interference
- shRNA:
-
small hairpin RNA
References
Huang Y, Mucke L . Alzheimer mechanisms and therapeutic strategies. Cell 2012; 148: 1204–1222.
Hardy J, Selkoe DJ . The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 2002; 297: 353–356.
Selkoe DJ . Alzheimer's disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis 2001; 3: 75–80.
Biswas SC, Shi Y, Vonsattel JP, Leung CL, Troy CM, Greene LA . Bim is elevated in Alzheimer's disease neurons and is required for beta-amyloid-induced neuronal apoptosis. J Neurosci 2007; 27: 893–900.
Kudo W, Lee HP, Smith MA, Zhu X, Matsuyama S, Lee HG . Inhibition of Bax protects neuronal cells from oligomeric Abeta neurotoxicity. Cell Death Dis 2012; 3: e309.
Troy CM, Rabacchi SA, Friedman WJ, Frappier TF, Brown K, Shelanski ML . Caspase-2 mediates neuronal cell death induced by beta-amyloid. J Neurosci 2000; 20: 1386–1392.
Putcha GV, Johnson EM Jr . Men are but worms: neuronal cell death in C elegans and vertebrates. Cell Death Differ 2004; 11: 38–48.
Gilley J, Coffer PJ, Ham J . FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol 2003; 162: 613–622.
Galili N, Davis RJ, Fredericks WJ, Mukhopadhyay S, Rauscher FJ 3rd, Emanuel BS et al. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet 1993; 5: 230–235.
Hillion J, Le Coniat M, Jonveaux P, Berger R, Bernard OA . AF6q21, a novel partner of the MLL gene in t(6;11)(q21;q23), defines a forkhead transcriptional factor subfamily. Blood 1997; 90: 3714–3719.
Anderson MJ, Viars CS, Czekay S, Cavenee WK, Arden KC . Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily. Genomics 1998; 47: 187–199.
Borkhardt A, Repp R, Haas OA, Leis T, Harbott J, Kreuder J et al. Cloning and characterization of AFX, the gene that fuses to MLL in acute leukemias with a t(X;11)(q13;q23). Oncogene 1997; 14: 195–202.
Corral J, Forster A, Thompson S, Lampert F, Kaneko Y, Slater R et al. Acute leukemias of different lineages have similar MLL gene fusions encoding related chimeric proteins resulting from chromosomal translocation. Proc Natl Acad Sci USA 1993; 90: 8538–8542.
Parry P, Wei Y, Evans G . Cloning and characterization of the t(X;11) breakpoint from a leukemic cell line identify a new member of the forkhead gene family. Genes Chromosomes Cancer 1994; 11: 79–84.
Jacobs FM, van der Heide LP, Wijchers PJ, Burbach JP, Hoekman MF, Smidt MP . FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J Biol Chem 2003; 278: 35959–35967.
Obsil T, Obsilova V . Structure/function relationships underlying regulation of FOXO transcription factors. Oncogene 2008; 27: 2263–2275.
Vogt PK, Jiang H, Aoki M . Triple layer control: phosphorylation, acetylation and ubiquitination of FOXO proteins. Cell Cycle 2005; 4: 908–913.
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999; 96: 857–868.
Greer EL, Brunet A . FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 2005; 24: 7410–7425.
Yang JY, Hung MC . A new fork for clinical application: targeting forkhead transcription factors in cancer. Clin Cancer Res 2009; 15: 752–757.
Fu Z, Tindall DJ . FOXOs, cancer and regulation of apoptosis. Oncogene 2008; 27: 2312–2319.
Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villén J, Becker EB et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell 2006; 125: 987–1001.
Yamagata K, Daitoku H, Takahashi Y, Namiki K, Hisatake K, Kako K et al. Arginine methylation of FOXO transcription factors inhibits their phosphorylation by Akt. Mol Cell 2008; 32: 221–231.
Yin KJ, Hsu CY, Hu XY, Chen H, Chen SW, Xu J et al. Protein phosphatase 2A regulates bim expression via the Akt/FKHRL1 signaling pathway in amyloid-beta peptide-induced cerebrovascular endothelial cell death. J Neurosci 2006; 26: 2290–2299.
Ham J, Towers E, Gilley J, Terzano S, Randall R . BH3-only proteins: key regulators of neuronal apoptosis. Cell Death Differ 2005; 12: 1015–1020.
Biswas SC, Shi Y, Sproul A, Greene LA . Pro-apoptotic Bim induction in response to nerve growth factor deprivation requires simultaneous activation of three different death signaling pathways. J Biol Chem 2007; 282: 29368–29374.
Lee HK, Kumar P, Fu Q, Rosen KM, Querfurth HW . The insulin/Akt signaling pathway is targeted by intracellular beta-amyloid. Mol Biol Cell 2009; 20: 1533–1544.
Frautschy SA, Baird A, Cole GM . Effects of injected Alzheimer beta-amyloid cores in rat brain. Proc Natl Acad Sci USA 1991; 88: 8362–8366.
Tiwari V, Patel AB . Impaired glutamatergic and GABAergic function at early age in AbetaPPswe-PS1dE9 mice: implications for Alzheimer's disease. J Alzheimers Dis 2012; 28: 765–769.
Al-Mubarak B, Soriano FX, Hardingham GE . Synaptic NMDAR activity suppresses FOXO1 expression via a cis-acting FOXO binding site: FOXO1 is a FOXO target gene. Channels (Austin) 2009; 3: 233–238.
Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M . APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature 2009; 457: 981–989.
Cuesto G, Enriquez-Barreto L, Carames C, Cantarero M, Gasull X, Sandi C et al. Phosphoinositide-3-kinase activation controls synaptogenesis and spinogenesis in hippocampal neurons. J Neurosci 2011; 31: 2721–2733.
Biswas SC, Liu DX, Greene LA . Bim is a direct target of a neuronal E2F-dependent apoptotic pathway. J Neurosci 2005; 25: 8349–8358.
Zhao Y, Wang Y, Zhu WG . Applications of post-translational modifications of FoxO family proteins in biological functions. J Mol Cell Biol 2011; 3: 276–282.
van der Horst A, Burgering BM . Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol 2007; 8: 440–450.
Matsuzaki H, Daitoku H, Hatta M, Tanaka K, Fukamizu A . Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc Natl Acad Sci USA 2003; 100: 11285–11290.
Yuan Z, Lehtinen MK, Merlo P, Villen J, Gygi S, Bonni A . Regulation of neuronal cell death by MST1-FOXO1 signaling. J Biol Chem 2009; 284: 11285–11292.
Sunters A, Madureira PA, Pomeranz KM, Aubert M, Brosens JJ, Cook SJ et al. Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res 2006; 66: 212–220.
Ho KK, McGuire VA, Koo CY, Muir KW, de Olano N, Maifoshie E et al. Phosphorylation of FOXO3a on Ser-7 by p38 promotes its nuclear localization in response to doxorubicin. J Biol Chem 2012; 287: 1545–1555.
Savage MJ, Lin YG, Ciallella JR, Flood DG, Scott RW . Activation of c-Jun N-terminal kinase and p38 in an Alzheimer's disease model is associated with amyloid deposition. J Neurosci 2002; 22: 3376–3385.
Graves JD, Gotoh Y, Draves KE, Ambrose D, Han DK, Wright M et al. Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like kinase Mst1. EMBO J 1998; 17: 2224–2234.
Qin W, Zhao W, Ho L, Wang J, Walsh K, Gandy S et al. Regulation of forkhead transcription factor FoxO3a contributes to calorie restriction-induced prevention of Alzheimer’s disease-type amyloid neuropathology and spatial memory deterioration. Ann N Y Acad Sci 2008; 1147: 335–347.
Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 2004; 305: 390–392.
Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004; 303: 2011–2015.
Picone P, Giacomazza D, Vetri V, Carrotta R, Militello V, San Biagio PL et al. Insulin-activated Akt rescues Abeta oxidative stress-induced cell death by orchestrating molecular trafficking. Aging Cell 2011; 10: 832–843.
Stohr O, Schilbach K, Moll L, Hettich MM, Freude S, Wunderlich FT et al. Insulin receptor signaling mediates APP processing and beta-amyloid accumulation without altering survival in a transgenic mouse model of Alzheimer’s disease. Age (Dordr) 2013; 35: 83–101.
Hong YK, Lee S, Park SH, Lee JH, Han SY, Kim ST et al. Inhibition of JNK/dFOXO pathway and caspases rescues neurological impairments in Drosophila Alzheimer's disease model. Biochem Biophys Res Commun 2012; 419: 49–53.
Keowkase R, Aboukhatwa M, Luo Y . Fluoxetine protects against amyloid-beta toxicity, in part via daf-16 mediated cell signaling pathway, in Caenorhabditis elegans. Neuropharmacology 2010; 59: 358–365.
Pallas M, Casadesus G, Smith MA, Coto-Montes A, Pelegri C, Vilaplana J et al. Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr Neurovasc Res 2009; 6: 70–81.
Barthelemy C, Henderson CE, Pettmann B . Foxo3a induces motoneuron death through the Fas pathway in cooperation with JNK. BMC Neurosci 2004; 5: 48.
You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M et al. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med 2006; 203: 1657–1663.
Nemoto S, Finkel T . Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science 2002; 295: 2450–2452.
Smith WW, Norton DD, Gorospe M, Jiang H, Nemoto S, Holbrook NJ et al. Phosphorylation of p66Shc and forkhead proteins mediates Abeta toxicity. J Cell Biol 2005; 169: 331–339.
Greene LA, Tischler AS . Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA 1976; 73: 2424–2428.
Park DS, Morris EJ, Padmanabhan J, Shelanski ML, Geller HM, Greene LA . Cyclin-dependent kinases participate in death of neurons evoked by DNA-damaging agents. J Cell Biol 1998; 143: 457–467.
Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, Janson B et al. Globular amyloid beta-peptide oligomer—a homogenous and stable neuropathological protein in Alzheimer's disease. J Neurochem 2005; 95: 834–847.
Rideout HJ, Wang Q, Park DS, Stefanis L . Cyclin-dependent kinase activity is required for apoptotic death but not inclusion formation in cortical neurons after proteasomal inhibition. J Neurosci 2003; 23: 1237–1245.
Biswas SC, Greene LA . Nerve growth factor (NGF) down-regulates the Bcl-2 homology 3 (BH3) domain-only protein Bim and suppresses its proapoptotic activity by phosphorylation. J Biol Chem 2002; 277: 49511–49516.
Wang Q, Maniati M, Jabado O, Pavlaki M, Troy CM, Greene LA et al. RAIDD is required for apoptosis of PC12 cells and sympathetic neurons induced by trophic factor withdrawal. Cell Death Differentiation 2006; 13: 75–83.
Acknowledgements
We thank Dr. KP Mohanakumar for allowing us to use the stereotaxic facility, and Mr. Raghavendra singh for his help in developing the animal model. We also thank Dr. AB Patel for providing tissues of transgenic animals of Alzheimer’s model and Mr. Vivek Tiwari for his help in processing the transgenic brains. We also thank Dr. PK Sarkar and Dr. Sumantra Das for their critical reading of this manuscript and their helpful discussions. This work was supported by one of the 12th Five-Year Plan Projects, miND (BSC0115) of CSIR, Government of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by D Bano
Supplementary Information accompanies this paper on Cell Death and Disease website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Sanphui, P., Biswas, S. FoxO3a is activated and executes neuron death via Bim in response to β-amyloid. Cell Death Dis 4, e625 (2013). https://doi.org/10.1038/cddis.2013.148
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2013.148
Keywords
This article is cited by
-
The APC/C E3 ligase subunit ANAPC11 mediates FOXO3 protein degradation to promote cell proliferation and lymph node metastasis in urothelial bladder cancer
Cell Death & Disease (2023)
-
The Molecular Effects of Environmental Enrichment on Alzheimer’s Disease
Molecular Neurobiology (2022)
-
Naturally-Occurring Antibodies Against Bim are Decreased in Alzheimer’s Disease and Attenuate AD-type Pathology in a Mouse Model
Neuroscience Bulletin (2022)
-
Role of FoxO transcription factors in aging and age-related metabolic and neurodegenerative diseases
Cell & Bioscience (2021)
-
BH3-only proteins Puma and Beclin1 regulate autophagic death in neurons in response to Amyloid-β
Cell Death Discovery (2021)