Abstract
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is best known for its selective cytotoxicity against transformed tumor cells. Most non-transformed primary cells and several cancer cell lines are not only resistant to death receptor-induced apoptosis, but also subject to inflammatory responses in a nuclear factor-κB (NF-κB)-dependent manner. Although the involvement of TRAIL in a variety of vascular disorders has been proposed, the exact molecular mechanisms are unclear. Here, we aimed to delineate the role of TRAIL in inflammatory vascular response. We also sought possible molecular mechanisms to identify potential targets for the prevention and treatment of post-angioplastic restenosis and atherosclerosis. Treatment with TRAIL increased the expression of intercellular adhesion molecule-1 by primary human vascular smooth muscle cells via protein kinase C (PKC)δ and NF-κB activation. Following detailed analysis using various PKCδ mutants, we determined that PKCδ activation was mediated by caspase-dependent proteolysis. The protective role of PKCδ was further confirmed in post-traumatic vascular remodeling in vivo. We propose that the TRAIL/TRAIL receptor system has a critical role in the pathogenesis of inflammatory vascular disorders by transducing pro-inflammatory signals via caspase-mediated PKCδ cleavage and subsequent NF-κB activation.
Similar content being viewed by others
Main
Abnormal proliferation of vascular smooth muscle cells (VSMCs) and accumulation of inflammatory cells are characteristic features of atherosclerosis and in-stent restenosis.1 Activated monocytes and macrophages secrete various pro-inflammatory cytokines and growth factors, which stimulate the migration and proliferation of VSMCs.2 Circumstantial evidence supports the involvement of tumor necrosis factor (TNF) superfamily members in this process.3, 4 The TNF superfamily of cytokines activates various signaling pathways involving cell survival, death, and differentiation, as well as inflammation and immune responses. Recently, TNF-related apoptosis-inducing ligand (TRAIL) was reported to exert biological responses other than apoptosis, such as the induction of pro-inflammatory responses by astrocytoma and the proliferation of VSMCs through activation of the nuclear factor-κB (NF-κB) pathway,5, 6, 7 although how TRAIL activates the NF-κB pathway in VSMCs is currently unclear. TNF-α and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), members of the TNF family of cytokines, have been implicated in the development of atherosclerosis;4, 8, 9 whereas genetic ablation of TRAIL has been shown to attenuate the development of atherosclerosis in apolipoprotein E-deficient mice.10
We have previously demonstrated that TRAIL and its receptors are involved in pro-angiogenic and pro-inflammatory signals of human astrocytoma cells.5, 11 Alternative functions of TRAIL and other TNF superfamily cytokines have been implicated in tumor evasion from host immune system.12 Considering the ubiquitous expression of TRAIL receptors, the TRAIL-TRAIL receptor system might be involved in a variety of pathological conditions such as atherosclerosis.4 In this study, we demonstrated that TRAIL can be pro-inflammatory and pro-atherogenic by showing that TRAIL ligation induces intercellular adhesion molecule-1 (ICAM-1) expression and subsequent monocytic adhesion by VSMCs via the activation of caspases and NF-κB. We also identified protein kinase C (PKC)δ as a molecular link between caspases and downstream NF-κB signals.
Results
TRAIL potentiates monocytic adhesion to VSMCs via the induction of ICAM-1 expression
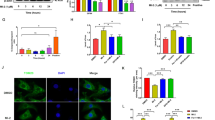
FACS analysis revealed that the VSMCs expressed all four TRAIL receptors (Figure 1a). Consistent with the in vitro data, the VSMCs of the pulmonary artery expressed TRAIL-R2/DR5, an agonistic TRAIL receptor, in vivo (Figure 1b). We investigated whether TRAIL acted as an inflammatory mediator in VSMCs. As determined by FACS analysis, we observed a marked increase in the level of ICAM-1 expression by VSMCs in response to TRAIL (Figure 1c). This effect was comparable to that of TNF-α, a well-known pro-inflammatory cytokine. TRAIL-mediated ICAM-1 expression was markedly inhibited by pretreatment with a soluble DR5 decoy receptor (Supplementary Figure 1). Treatment with TRAIL or TNF-α significantly increased monocytic adhesion to VSMCs (Figure 1d), consistent with the increase in ICAM-1 expression. Blockade of CD18 completely inhibited monocytic adhesion to VSMCs in response to TRAIL stimulation, indicating that ICAM-1 and (lymphocyte function-associated antigen-1 or CD11a/CD18) or Mac-1 (CD11b/CD18) interactions have key roles in TRAIL-mediated monocytic adhesion.
TRAIL-induced monocytic adhesion to VSMCs via the expression of ICAM-1. (a) VSMCs were examined by flow cytometry for the expression of TRAIL receptors. (b) A normal human pulmonary artery was stained for TRAIL-R2, and nuclei were counterstained with hematoxylin. Scale bar=100 μm. (c) Cells were incubated with TRAIL (500 μg/l) or TNF-α (10 μg/l) for 24 h and ICAM-1 expression was evaluated by flow cytometry. MFI, mean fluorescence intensity. (d) VSMCs were incubated in the absence or presence of TRAIL and TNF-α for 24 h, incubated with anti-CD18 antibody for an additional 1 h, and then stained with Hoechst 33258. Activated U937 cells were allowed to adhere to the VSMCs. A P-value is indicated when the difference was statistically significant (Student's t-test). (e) VSMCs were incubated in the absence or presence of MG-132 or zVAD-fmk (zVAD; 20 μmol/l) for 1 h and then treated with TRAIL for various time periods. Cell lysates were examined by immunoblot analysis for caspase-3. β-Actin protein was used as a loading control
We next evaluated the ability of TRAIL to induce apoptosis in VSMCs. Treatment with TRAIL scarcely induced apoptotic cell death of VSMC. Although TRAIL did not induce significant cell death, the active fragment of caspase-3 was detected within 1 h of treatment with TRAIL by immunoblot analysis. Treatment with MG-132, a proteasome inhibitor that accumulates active caspase-3,13 markedly enhanced the TRAIL-mediated cleavage of caspase-3 (Figure 1e) and subsequent cell death (Supplementary Figure 2). The cleavage of active caspase-3 was completely abrogated by pretreatment with zVAD-fmk. Collectively, these results indicate that the death domain-containing TRAIL receptors expressed by VSMCs do not induce apoptotic cell death, but rather pro-inflammatory responses, even though TRAIL ligation triggers caspase-3 activation.
TRAIL induces the activation of NF-κB and subsequent expression of ICAM-1 in a caspase-dependent manner.
Because TRAIL treatment induced caspase-3 cleavage in the absence of overt cytotoxicity, we next examined the involvement of caspases in TRAIL-induced ICAM-1 expression. Enhanced expression of ICAM-1 upon TRAIL stimulation was significantly blocked by pretreatment with a broad-spectrum caspase inhibitor zVAD-fmk, as well as specific inhibitors for caspase-8 and -3 (i.e. zIETD-fmk and zDEVD-fmk, respectively; Figure 2a). In contrast, pretreatment with zVAD-fmk did not inhibit TNF-α-induced expression of ICAM-1 in VSMCs.
TRAIL induced the activation of NF-κB and subsequent expression of ICAM-1 in a caspase-dependent manner. (a) Cells were incubated in the absence or presence of the caspase inhibitors zVAD-fmk, zIETD-fmk (zIETD), and zDEVD-fmk (zDEVD) (20 μmol/l) for 1 h, and then treated with TRAIL or TNF-α for an additional 24 h. ICAM-1 protein was measured by flow cytometry. (*) a significant difference compared with the sample treated with TRAIL alone. *P<0.0001, **P=0.002, ***P=0.034; Tukey’s post-hoc test was applied to significant group effects in analysis of variance (ANOVA), F6, 19=51.234, P<0.0001 for the flow cytometric analysis. (b) Cells were incubated in the absence or presence of the caspase inhibitor zVAD-fmk (zVAD; 20 μmol/l) for 1 h, pretreated with SN-50 (50 mg/l) or lactacystin (10 μmol/l) for 1 h, and then treated with TRAIL for 24 h. ICAM-1 protein was measured by flow cytometry. (*) a significant difference compared with the sample treated with TRAIL alone. *P=0.001, **P<0.0001; Tukey’s post-hoc test was applied to significant group effects in ANOVA (F8,9=40.456, P<0.0001) for the flow cytometric analysis. (c) VSMCs were incubated in the absence or presence of zVAD-fmk (zVAD; 20 μmol/l) for 1 h and treated with trail or TNF-α for various time periods (0–60 min); then cell lysates were analyzed for p-IKK, IKK, p-IκBα, and IκBα by immunoblotting. (d) VSMCs were incubated in the absence or presence of zVAD-fmk, SN-50 (50 mg/l), and lactacystin (lacta; 10 μmol/l), then treated with TRAIL or TNF-α for 60 or 30 min, respectively. Cells were stained with an anti-p65 antibody via an immunocytochemical method
To determine whether caspase cascades are involved in NF-κB activation, we tested the combined effects of two different inhibitors (i.e. zVAD-fmk+NF-κB inhibitor) on ICAM-1 expression. As shown in Figure 2b, suppression of TRAIL-mediated ICAM-1 expression by zVAD-fmk was not further affected by addition of NF-κB inhibitors such as SN-50 or lactacystin. Cells pretreated with zVAD-fmk showed a significant decrease in phosphorylation of IκB kinase (IKK) and inhibitor of κB (IκB), which are important steps in the activation of NF-κB (Figure 2c). In contrast, TNF-α-induced phosphorylation of these molecules was not affected by pretreatment with zVAD-fmk. Treatment of VSMCs with TRAIL or TNF-α induced the degradation of IκBα and the subsequent nuclear translocation of the NF-κB p65 subunit within 60 min following treatment (Figure 2d). The extent of p65 translocation into the nucleus in response to TRAIL decreased significantly in the presence of zVAD-fmk or NF-κB inhibitors such as SN-50 and lactacystin. In the case of TNF-α, p65 translocation was significantly blocked only by NF-κB inhibitors, but not caspase inhibitors.
PKCδ acts downstream of caspases in the TRAIL-mediated NF-κB pathway
Various studies have demonstrated a critical role of PKC isoforms in the NF-κB pathway at the level of IKK activation and IκB degradation.14, 15. Human VSMCs expressed considerably high levels of PKCα and PKCδ; whereas PKCβ isoform was not detected by immunoblot analysis (Supplementary Figure 3a). Cells transfected with PKCδ siRNA displayed a significant reduction in TRAIL-induced phosphorylation of IKK and the subsequent phosphorylation of IκB; whereas transfection with control siRNA or siRNAs specific for PKCα or PKCβ had little effect on TRAIL-induced phosphorylation of IKK and IκB (Figure 3a and Supplementary Figure 3b). Because PKCδ is known to be proteolytically activated by caspases, we next determined whether TRAIL ligation could activate PKCδ through cleavage by caspases. Immunoblot analysis demonstrated that TRAIL ligation induced a time-dependent proteolytic cleavage of PKCδ to produce a short carboxy-terminus fragment (41 kDa; Supplementary Figure 4). The caspase-dependent cleavage of PKCδ was further confirmed by introducing a PKCδD329A construct, which has a mutation in the caspase cleavage site (Figure 3b).
Caspase-dependent cleavage of PKCδ is responsible for TRAIL-induced NF-κB activation. (a) VSMCs transfected with scrambled control siRNA or PKC-specific siRNA were treated with TRAIL for various time periods (0–90 min) and the cell lysates were analyzed for p-IKK, IKK, p-IκBα, IκBα, PKCδ, and β-actin by immunoblotting. A representative of two independent experiments is shown. (b) VSMCs were transfected with PKCδ constructs (PKCδ wild-type (WT); PKCδ D394A, DA; PKCδ-CF, CF) for 16 h and then treated with TRAIL for 4 h. Cell lysates were subjected to immunoblot analysis for Flag, PKCδ, and β-actin. (c) VSMCs were transfected with PKCδ constructs (PKCδ WT; PKCδ-CF, CF) and a NF-κB luciferase reporter plasmid for 16 h and treated with TRAIL for 6 h. Cells were lysed and a luciferase assay was performed. A P-value is indicated (Student’s t-test). Samples significantly different from the control sample were indicated with symbols (*P<0.01; **P<0.001). Data indicate the mean of four samples from a representative among three independent experiments. (d) VSMCs were transfected with PKCδ constructs (PKCδ WT; PKCδ D394A, DA) and a NF-κB luciferase reporter plasmid for 16 h and treated with TRAIL or TNF-α for 6 h. Cells were lysed and a luciferase assay was performed. A P-value is indicated (Student’s t-test). Samples significantly different from the control sample were indicated with symbols (*P<0.01; **P<0.001). Data indicate the mean of four samples from a representative among four independent experiments. (e) VSMCs were transfected with Nox4 siRNA and a NF-κB luciferase reporter plasmid for 24 h and treated with TRAIL or TNF-α for 6 h. Cells were lysed and a luciferase assay was performed. A P-value is indicated when the difference was statistically significant (Student's t-test). The Nox4 mRNA level was determined by qPCR. (f) Cells transfected with scrambled control siRNA or Nox4-specific siRNA were treated with TRAIL or TNF-α for 24 h. ICAM-1 protein was measured by flow cytometry. A P-value is indicated when the difference was statistically significant (Student's t-test). (g) Transfection mixtures of control siRNA or PKCδ siRNA were injected along the carotid artery after balloon injury of the artery. Ten days after the injury, the arterial tissue was isolated and stained with hematoxylin and eosin. The intimal and medial areas were determined and the ratio of intima to media was calculated
To determine whether caspase-dependent cleavage of PKCδ is required for TRAIL-induced NF-κB activation, we performed a NF-κB reporter assay following the introduction of wild-type PKCδ and PKCδ D394A constructs. As expected, TRAIL but not TNF-α failed to increase the NF-κB reporter activity after the introduction of the PKCδ D394A construct (Figure 3c). To directly confirm the role of active PKCδ fragment released by proteolytic cleavage, we generated a truncation mutant representing the caspase-cleaved catalytic fragment of PKCδ, so-called PKCδ-CF. The introduction of PKCδ-CF significantly increased the NF-κB activation as compared with the construct control or PKCδ wild-type; whereas TRAIL ligation further increased the NF-κB activity (Figure 3d). These results clearly demonstrate that the caspase-dependent generation of catalytic fragment of PKCδ is required for TRAIL-induced NF-κB activation.
Although PKCs are known to directly phosphorylate the IKK responsible for NF-κB activation, it is also proposed that NAD(P)H oxidase (Nox) regulation of PKCs leads to NF-κB activation through reactive oxygen species (ROS) generation.16 Because we have observed the proteolytic cleavage of PKCδ and generation of intracellular ROS upon TRAIL ligation (Supplementary Figure 5), we investigated the involvement of Nox in the NF-κB pathway. We examined the knockdown effects of Nox4, a major vascular Nox subunit, on NF-κB activation and subsequent ICAM-1 expression. Cells transfected with Nox4 siRNA exhibited a significant reduction in TRAIL-induced activity of the NF-κB reporter gene, indicating that the Nox4 isoform mediates NF-κB activation induced by TRAIL (Figure 3d). In addition, Nox4 knockdown significantly suppressed TRAIL-induced ICAM-1 expression (Figure 3e). The NF-κB activation and subsequent ICAM-1 expression induced by TNF-α also suppressed by Nox4 siRNA transfection are consistent with a previous report stating that Nox facilitates the redox-dependent induction of NF-κB by TNF-α.17 Knockdown of Nox1, another vascular Nox subnit, suppressed only TNF-α-induced ICAM-1 expression but not that induced by TRAIL ligation, suggesting the differential involvement of Nox isoform (Supplementary Figure 6).
The observations described here suggest that PKCδ has a critical role in NF-κB-mediated ICAM-1 expression induced by the pro-inflammatory cytokine, TRAIL. Therefore, to demonstrate the involvement of PKCδ in post-traumatic vascular remodeling process, in which an acute pro-inflammatory response is critical, neointimal growth was analyzed following the in vivo transfection of PKCδ siRNA in an arterial injury model. Quantitative histomorphometric analysis clearly showed a significant reduction in the neointimal/medial area ratio in PKCδ siRNA-transfected vessels as compared with control vessels (Figure 3f).
Discussion
Current findings suggest a novel role of PKCδ in the TRAIL-mediated signal transduction pathway that leads to caspase-dependent NF-κB activation by VSMCs (Figure 4). We confirmed that TRAIL ligation induced expression of the NF-κB-responsive pro-inflammatory gene, ICAM-1, which has an important role in the regulation of inflammatory responses. Even though TRAIL ligation can also induce ICAM-1 expression by human astrocytoma cells in a similar manner, the major role of TRAIL system is to induce an apoptotic cell death, which can be regulated by various intracellular signaling such as the Nox-ROS pathways.18, 19 On the contrary, the limited potential of TRAIL to induce apoptotic cell death suggests the involvement of the TRAIL system in the pathogenesis of inflammatory vascular disorders including atherosclerosis and post-angioplastic restenosis.7, 9 This study prompts further evaluation of TRAIL and PKCδ as novel therapeutic targets for development of medications to treat chronic inflammatory vascular disorders.
Recent reports suggest non-apoptotic roles of caspases by cleavage of unidentified intermediates so as to transduce non-apoptotic signals.20, 21 This hypothesis is supported by the observation that caspase-8 is involved in the cleavage of IκB between Asp31 and Ser,32 leading to the activation of NF-κB.22 Another study demonstrated that IKK complex recruitment and activation, as well as the nuclear translocation of NF-κB, require the enzymatic activity of full-length caspase-8, and that caspase-8 deficiency abolishes the activation of NF-κB after stimulation through Toll-like receptors in immune cells.23, 24 Recent evidence also suggests that PKCδ acts as a pro-survival factor in the process of apoptosis,25, 26, 27 although numerous reports have revealed a role for PKCδ as a mediator of the cell death program. SMCs of the expanding neointima from PKCβ-null mice were growth restricted whereas PKCδ-null mice exhibited significantly higher numbers of SMCs, supporting the notion that PKCδ generally suppresses normal cell proliferation.28 In contrast, our results showed that transfection of PKCδ siRNA significantly reduced the neointimal thickness, suggesting that the PKCδ-mediated inflammatory response is crucial to post-traumatic vascular remodeling in response to acute injury. Collectively, these observations indicate that PKCδ and caspases might have distinct functions in the regulation of cell proliferation and apoptosis in a context-dependent manner.
Despite the fact that caspase activation is a key feature of TRAIL signaling in VSMCs, we did not observe TRAIL-induced apoptotic cell death in VSMCs. The resistance of VSMCs to TRAIL-mediated apoptosis can be explained by several mechanisms. First, sensitivity to TRAIL-mediated apoptosis can be determined at the receptor level.29 TRAIL, either as a type II transmembrane protein or as a soluble protein, interacts with four different transmembrane receptors, namely TRAIL-R1 and TRAIL-R2 (which transmit apoptotic signals) and TRAIL-R3 and TRAIL-R4 (which lack a functional death domain and therefore function as decoy receptors to protect cells from apoptosis).30 According to our results, TRAIL-R2 is expressed most highly by VSMCs, and the levels of TRAIL-R3 and -R4 are negligible in TRAIL-resistant VSMCs. Therefore, we can exclude the increased expression of decoy receptors as a possible mechanism for TRAIL resistance of VSMCs. Second, high levels of cellular FLICE inhibitory protein (c-FLIP) or X-linked inhibitor of apoptosis protein (XIAP), endogenous inhibitors of caspase-8 and -3, may be responsible for resistance to TRAIL-mediated cell death.31, 32 Some studies have shown an increased expression of FLIP by Fas-resistant SMCs.33 However, increased levels of c-FLIP or XIAP block not only TRAIL-mediated cell death, but also the activation of caspase itself. We can exclude the possibility that the TRAIL-resistant mechanism is mediated by c-FLIP or XIAP expression because TRAIL ligation induces the activation of caspase-3 in VSMCs.
Our results demonstrated that proteasome inhibition sensitized VSMCs to TRAIL-induced cell death. Treatment with TRAIL scarcely induced VSMC cell death, whereas this effect was markedly enhanced by the presence of the protein synthesis inhibitor cycloheximide or the proteasome inhibitor MG-132 (Supplementary Figure 4A). Cells pretreated with the proteasome inhibitor lactacystin showed a similar pattern of caspase cleavage as cells pretreated with MG-132 (Supplementary Figure 4B). In contrast, cells pretreated with SN-50, which inhibits nuclear translocation of NF-κB, showed no increase in the active form of caspase-3 in the presence of TRAIL. These findings suggest that NF-κB inhibition by SN-50 may not affect caspase-3 activation, but rather proteasome inhibition by MG-132 or lactacystin may increase the active form of caspase-3 by stabilizing the molecule.13 These results revealed that accumulated active caspase-3 by proteasomal inhibition can trigger apoptotic cell death in TRAIL signaling, whereas the death domain-containing TRAIL receptors expressed by VSMCs do not cause apoptotic cell death, even though TRAIL ligation induces caspase-3 activation.
Recent evidence suggests that TRAIL promotes the proliferation of VSMCs via activation of the NF-κB pathway,7 although how TRAIL activates the NF-κB pathway in VSMCs is currently unclear. The results described here suggest the involvement of caspase-dependent PKCδ activation in the TRAIL-mediated NF-κB pathway. Although a mechanism for direct NF-κB activation induced by the upstream PKC exists, we tested the additional involvement of a Nox isoform in the NF-κB pathway based on the rationale that Nox-dependent ROS generation is also important for NF-κB activation. The concept that ROS regulate gene expression appears to be generally accepted, particularly in regard to NF-κB activation by pro-inflammatory cytokines.34 To speculate that the expression of the pro-inflammatory gene ICAM-1 following TRAIL stimulation is regulated by intracellular ROS is tempting. This hypothesis is supported by the finding that TRAIL produces intracellular ROS in a time-dependent manner (Supplementary Figure 5) and that TRAIL-induced NF-κB activation and subsequent ICAM-1 expression are suppressed by Nox4 siRNA transfection (Figures 3d and e). A mechanism through which an increase in ROS level following TRAIL stimulation could affect the activation of NF-κB remains to be clarified. Given that many kinases in the NF-κB pathway are redox sensitive, ROS may activate NF-κB by regulating upstream signaling, which leads to the phosphorylation of IκB. Alternatively, the NF-κB subunit itself, as well as the upstream kinase of IκB in the NF-κB pathway, may be directly modified by ROS.35 In addition, we observed a differential involvement of Nox isoforms in ICAM-1 expression. Nox4 knockdown significantly suppressed TRAIL-induced ICAM-1 expression, whereas Nox1 siRNA had an inhibitory effect only on TNF-α-induced ICAM-1 expression (Figure 3e and Supplementary Figure 6). These results suggest that Nox1 and Nox4 differentially regulate the TRAIL and TNF-α-signaling pathways; Nox1 is only involved in the TNF-α-mediated signaling pathway, whereas Nox4 affects both TRAIL and TNF-α signaling.
ROS, such as superoxide anions and hydrogen peroxide, are generated inside cells in response to physiological stimulation by growth factors and cytokines.36 Evidence suggests that ROS are responsible for cytokine-induced NF-κB activation, although relatively little is known regarding the molecular mechanisms by which cytokine stimulation might increase ROS levels and subsequent NF-κB activation.37 The multicomponent enzyme Nox is regarded as a major source of ROS responsible for various cell signaling. Its activation is regulated by the phosphorylation of cytosolic components, which is mainly mediated by several PKC isoforms.38 Among these, PKCδ is ubiquitously expressed as a Ser/Thr kinase of the PKC superfamily and has been implicated in the control of wide physiological responses, including VSMC homeostasis.39
Nox has emerged as a major source of ROS in the cardiovascular system, although the precise molecular mechanisms responsible for receptor-mediated generation of ROS are not fully understood. Most vascular cells express multiple Nox proteins, including gp91phox (also known as Nox2), Nox1, Nox4 and Nox5.36 Of these, Nox4, which is strongly expressed in all cells of the vascular wall, appears to be important for ligand-induced production of hydrogen peroxide.40 Nonetheless, little is known regarding the specific mechanism of Nox4 activation. Previous studies have demonstrated that PKCδ serves as a key substrate for caspase-3 during apoptotic cell death.41 Caspase-3 cleaves the kinase to yield a 41-kDa catalytically active fragment and a 37-kDa regulatory fragment, which continuously activate the kinase.41 We hypothesized a potential mechanism in which caspase regulation of ROS generation occurs through caspase-dependent activation of PKCδ. As expected, caspase was found to be essential for PKCδ cleavage, which induces an increase in ROS and subsequent ICAM-1 expression, thereby indicating that PKCδ acts as a link between caspase and Nox activation for NF-κB activation. At the present time, Nox4 has no known regulatory subunit, so how the cleaved PKCδ affects the activation of Nox4 remains to be discovered. Recently, a mechanism for p47phox phosphorylation in the activation of Nox isoforms was reported.42 This raises the possibility that p47phox is involved in PKC-mediated activation of Nox upon TRAIL stimulation.
Materials and Methods
Cell culture and reagents
Human aortic smooth muscle cells were purchased from LONZA (Walkersville, MD, USA) and cultured in the medium provided by the company. The experiments were performed with cells that were cultured for six to seven passages. Human recombinant TRAIL and TNF-α were obtained from R&D Systems (Minneapolis, MN, USA). TRAIL-R2/DR5 response was examined using soluble DR5:Fc chimera (Alexis corporation, San Diego, CA, USA). Cycloheximide and MG-132 were purchased from Calbiochem (La Jolla, CA, USA). NF-κB inhibitors including SN-50 and lactacystin were from Biomol Research Laboratories (Plymouth Meeting, PA, USA), and Calbiochem, respectively. Rottlerin and the caspase inhibitors, which included zVAD-fmk, zIETD-fmk, and zDEVD-fmk, were purchased from Calbiochem.
Flow cytometric analysis
For analysis of TRAIL receptors expression, VSMCs were trypsinized, suspended in PBS that contained 3% fetal bovine serum, and incubated with anti-human TRAIL-R1, TRAIL-R2, TRAIL-R3, or TRAIL-R4 antibody for 1 h (Alexis Biochemicals, Lausanne, Switzerland). VSMCs were washed and stained with phycoerythrin (PE)-conjugated anti-mouse IgG1 (BD Biosciences, San Jose, CA, USA) for an additional 1 h, washed again, and then analyzed by FACStar (BD Biosciences). A PE-conjugated, isotype-matched (IgG1) antibody (BD Biosciences) was used as a negative control. Within 30 min of staining, 10 000 cells were analyzed by FACStar .
For analysis of ICAM-1 expression, VSMCs were trypsinized, suspended in PBS that contained 3% fetal bovine serum, and incubated with PE-conjugated anti-ICAM-1 antibody (BD Biosciences). VSMCs were washed and stained with phycoerythrin (PE)-conjugated anti-mouse IgG1 (BD Biosciences) for an additional 1 h, washed again, and then analyzed by FACStar. A PE-conjugated, isotype-matched (IgG1) antibody (BD Biosciences) was used as a negative control. Cell death was determined by staining with Annexin V-fluorescein isothiocyanate and propodium iodide (BD Biosciences), as described previously.5 Within 30 min of staining, 10 000 cells were analyzed by FACStar .
Immunohistochemistry
Normal human pulmonary arteries were embedded in paraffin and cut into 5-μm-thick sections. The specimens were washed with PBS and incubated for 1 h with mouse anti-TRAIL-receptors antibodies, which were pre-diluted in PBS with horse serum. Biotin-labeled secondary antibodies were applied for 30 min, followed by avidin–biotin–peroxidase complex (ABC kit; Vector Laboratories, Burlingame, CA, USA). Normal mouse IgG1 (Clontech, Palo Alto, CA, USA) was used as the control. Cell nuclei were counterstained with hematoxylin. The study using human pulmonary artery conforms with the principles outlined in the Declaration of Helsinki and was approved by the Institutional Review Board of Mokdong Medical Center (Ewha Womans University).
Adhesion assay
Human monocytic U937 cells were activated with lipopolysaccharide (100 μg/l) for 24 h, and stained with 1 μmol/l TMRE (Invitrogen, Carlsbad, CA, USA) for 10 min. The U937 cells were then allowed to adhere to VSMCs. After 30 min, the cells were washed to remove non-adherent U937 cells. Adherent U937 cells were analyzed under an inverted fluorescence microscope. In the blocking experiments, U937 cells were pre-incubated with anti-CD18 antibody (Calbiochem) for 1 h before cell adhesion. Mouse IgG1 was used as an isotype control antibody (BD Biosciences).
Western blot analysis
Total cell extracts (20 μg of total protein) were prepared and electrophoresed in 12% SDS gels, as described previously.5 The proteins were then transferred to nitrocellulose membranes and probed with antibodies directed against human caspase-3, DYKDDDDK (Flag) tag, phospho-IKK (p-IKK), IKK, phospho-IκBα (p-IκBα), and IκBα (Cell Signaling Technology, Beverly, MA, USA). The anti-PKCδ and anti-β-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Enhanced chemiluminescence was used to detect the bound antibodies (AbFrontier, Seoul, Korea).
Plasmids, siRNA, and cell transfection
Transfection was performed using Lipofectamine(Invitrogen), according to the manufacturer's protocol. The siRNA oligonucleotides that encode Nox1, Nox4, and PKCδ were obtained from Bioneer (Daejeon, Korea), and the sequences were described in Supplementary Table 1 (Bioneer). At 48 h post-transfection, total RNA was extracted from transfected cells using the Trizol reagent (Gibco BRL, Grand Island, NY, USA). The cDNA derived from 1 μg of total RNA was subjected to RT-PCR with the following Nox4-specific primers (Supplementary Table 2). Conventional PCR reactions were performed using an initial denaturation step at 95°C (5 min) followed by 23 cycles of 94°C (20 s), 56°C (30 s), and 72°C (30 s). Quantitative PCR was performed with a real-time PCR system (Applied Biosystems, CA, USA). Each reaction was done in triplicate using a reaction solution containing SYBR Green buffer and the primers (0.3 mmol/l, Supplementary Table 3). The mRNA level was normalized by that of GAPDH.
Human wild-type PKCδ was cloned into pCMV-3Tag-3A, and a mutation in the caspase cleavage site (pCMV-3Tag-PKCδD-A329) was generated from pCMV-3Tag-PKCδ by PCR site-directed mutagenesis using a Quick Change site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) according to the manufacturer's protocol. The catalytic fragment of wild-type PKCδ was generated by PCR using full-length pCMV-3Tag-PKCδ as a template, starting at the caspase cleavage site (amino acid residue 378).43 For NF-κB reporter assay, cells were transfected with the reporter plasmids NF-κB luciferase by using Lipofectamine and then treated with TRAIL or TNF-α. After 6 h treatment cells were lysed according to the luciferase assay system with lysis buffer protocol (Promega, Madison, WI, USA). Luminescence from the lysates was measured by Wallac multilabel counter (PerkinElmer Wallac, Gaithersburg, MD, USA).
Nuclear location of p65NF-κB by immunocytochemistry
Nuclear translocation of p65 was observed by the immunocytochemical method. Treated cells were fixed with 4% paraformaldehyde, and permeabilized with 0.2% of Triton X-100 (Sigma, St. Louis, MO, USA). After being washed in PBS, slides were blocked with 2% bovine albumin serum for 1 h and then incubated with rabbit polyclonal anti-human p65 (Santa Cruz) at 1 : 100 dilutions. After overnight incubation at 4°C, the slides were washed and incubated with goat anti-rabbit IgG-fluorescein isothiocyanate (Santa Cruz) at 1 : 500 dilutions for 1 h.
Rat balloon-injured model
A balloon injury was created with an infiltrated 2F Fogarty balloon catheter (ACS RX Comet, Advanced Cardiovascular System, Inc., Temecula, CA, USA) in the normal left rat carotid artery as described previously.44 Ten-week-old male Sprague-Dawley rats were anaesthetized, the left external carotid artery was exposed, and its branches were electro-coagulated. A catheter was pushed 1 cm in through the transverse arteriotomy of the external carotid artery, and the endothelial denudation was achieved by three passes along the common carotid artery. Transfection mixtures (PKCδ siRNA or Control siRNA, 200 nmol/l) were prepared using siPORT NeoFX reagent following the manufacturer's instructions (Ambion, Austin, TX, USA). Balloon-injured region was washed with 200 μl opti-MEM by punched balloon catheter. Each transfection mixtures in punched balloon catheter were injected along the common carotid artery. After 15 min incubation, blood flow in occluded left common carotid artery was reperfused. Rottlerin (50 μmol/l) in situ transfection experiment was accomplished in similar procedures. One week or 10 days after the injury and siRNA transfection, the common carotid arteries were excised after transcardiac perfusion fixation with heparinized saline containing 3.7% formaldehyde, and paraffin embedded. Five serial tissue sections (100-μm interval and 4-μm thickness) were obtained from the middle area of common carotid arteries. Each slide was stained with haematoxylin and eosin. All experiments were conducted in compliance with the Guide for the Care and Use of Laboratory Animals of Ewha Women's University, Seoul, Korea. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85-23, revised 1996).
Statistical analysis
Data are presented as the mean±S.D. Levels of significance for comparisons between two independent samples were determined using the Student’s t-test. Groups were compared by analysis of variance with Tukey’s honestly significant difference post-hoc test applied to significant main effects (SPSS 12.0 K for Windows; SPSS Inc., Chicago, IL, USA).
Abbreviations
- ICAM-1:
-
intercellular adhesion molecule-1
- IKK:
-
IκB kinase
- NF-κB:
-
nuclear factor-κB
- NOX:
-
NAD(P)H oxidase
- PKC:
-
protein kinase C
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- TNF:
-
tumor necrosis factor
- VSMC:
-
vascular smooth muscle cell
References
Brooks G, Yu XM, Wang Y, Crabbe MJ, Shattock MJ, Harper JV . Non-steroidal anti-inflammatory drugs (NSAIDs) inhibit vascular smooth muscle cell proliferation via differential effects on the cell cycle. J Pharm Pharmacol 2003; 55: 519–526.
Wen JK, Han M, Zheng B, Yang SL . Comparison of gene expression patterns and migration capability at quiescent and proliferating vascular smooth muscle cells stimulated by cytokines. Life Sci 2002; 70: 799–807.
Aggarwal BB, Shishodia S, Ashikawa K, Bharti AC . The role of TNF and its family members in inflammation and cancer: lessons from gene deletion. Curr Drug Targets Inflamm Allergy 2002; 1: 327–341.
Michowitz Y, Goldstein E, Roth A, Afek A, Abashidze A, Ben Gal Y et al. The involvement of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in atherosclerosis. J Am Coll Cardiol 2005; 45: 1018–1024.
Choi C, Kutsch O, Park J, Zhou T, Seol DW, Benveniste EN . Tumor necrosis factor-related apoptosis-inducing ligand induces caspase-dependent interleukin-8 expression and apoptosis in human astroglioma cells. Mol Cell Biol 2002; 22: 724–736.
Secchiero P, Gonelli A, Carnevale E, Milani D, Pandolfi A, Zella D et al. TRAIL promotes the survival and proliferation of primary human vascular endothelial cells by activating the Akt and ERK pathways. Circulation 2003; 107: 2250–2256.
Kavurma MM, Schoppet M, Bobryshev YV, Khachigian LM, Bennett MR . TRAIL stimulates proliferation of vascular smooth muscle cells via activation of NF-kappaB and induction of insulin-like growth factor-1 receptor. J Biol Chem 2008; 283: 7754–7762.
Boyle JJ . Macrophage activation in atherosclerosis: pathogenesis and pharmacology of plaque rupture. Curr Vasc Pharmacol 2005; 3: 63–68.
Chan J, Prado-Lourenco L, Khachigian LM, Bennett MR, Di Bartolo BA, Kavurma MM . TRAIL promotes VSMC proliferation and neointima formation in a FGF-2-, Sp1 phosphorylation-, and NFkappaB-dependent manner. Circ Res 2010; 106: 1061–1071.
Watt V, Chamberlain J, Steiner T, Francis S, Crossman D . TRAIL attenuates the development of atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis 2011; 215: 348–354.
Choi K, Song S, Choi C . Requirement of caspases and p38 MAPK for TRAIL-mediated ICAM-1 expression by human astroglial cells. Immunol Lett 2008; 117: 168–173.
Choi C, Benveniste EN . Fas ligand/Fas system in the brain: regulator of immune and apoptotic responses. Brain Res Rev 2004; 44: 65–81.
Kim S, Choi K, Kwon D, Benveniste EN, Choi C . Ubiquitin-proteasome pathway as a primary defender against TRAIL-mediated cell death. Cell Mol Life Sci 2004; 61: 1075–1081.
Lallena MJ, Diaz-Meco MT, Bren G, Paya CV, Moscat J . Activation of IkappaB kinase beta by protein kinase C isoforms. Mol Cell Biol 1999; 19: 2180–2188.
Spitaler M, Cantrell DA . Protein kinase C and beyond. Nat Immunol 2004; 5: 785–790.
Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T et al. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J Biol Chem 2003; 278: 25234–25246.
Li Q, Spencer NY, Oakley FD, Buettner GR, Engelhardt JF . Endosomal Nox2 facilitates redox-dependent induction of NF-kappaB by TNF-alpha. Antioxid Redox Signal 2009; 11: 1249–1263.
Choi K, Choi C . Proapoptotic ginsenosides compound K and Rh enhance Fas-induced cell death of human astrocytoma cells through distinct apoptotic signaling pathways. Cancer Res Treat 2009; 41: 36–44.
Choi K, Han YH, Choi C . N-acetyl cysteine and caffeic acid phenethyl ester sensitize astrocytoma cells to Fas-mediated cell death in a redox-dependent manner. Cancer Lett 2007; 257: 79–86.
Sakamaki K, Inoue T, Asano M, Sudo K, Kazama H, Sakagami J et al. Ex vivo whole-embryo culture of caspase-8-deficient embryos normalize their aberrant phenotypes in the developing neural tube and heart. Cell Death Differ 2002; 9: 1196–1206.
Carlile GW, Smith DH, Wiedmann M . Caspase-3 has a nonapoptotic function in erythroid maturation. Blood 2004; 103: 4310–4316.
Rathore N, Matta H, Chaudhary PM . An evolutionary conserved pathway of nuclear factor-kappaB activation involving caspase-mediated cleavage and N-end rule pathway-mediated degradation of IkappaBalpha. J Biol Chem 2004; 279: 39358–39365.
Su H, Bidere N, Zheng L, Cubre A, Sakai K, Dale J et al. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science 2005; 307: 1465–1468.
Lemmers B, Salmena L, Bidere N, Su H, Matysiak-Zablocki E, Murakami K et al. Essential role for caspase-8 in Toll-like receptors and NFkappaB signaling. J Biol Chem 2007; 282: 7416–7423.
Ryer EJ, Sakakibara K, Wang C, Sarkar D, Fisher PB, Faries PL et al. Protein kinase C delta induces apoptosis of vascular smooth muscle cells through induction of the tumor suppressor p53 by both p38-dependent and p38-independent mechanisms. J Biol Chem 2005; 280: 35310–35317.
Reyland ME . Protein kinase Cdelta and apoptosis. Biochem Soc Trans 2007; 35 (Part 5): 1001–1004.
Zhang J, Liu N, Zhang J, Liu S, Liu Y, Zheng D . PKCdelta protects human breast tumor MCF-7 cells against tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis. J Cell Biochem 2005; 96: 522–532.
Leitges M, Mayr M, Braun U, Mayr U, Li C, Pfister G et al. Exacerbated vein graft arteriosclerosis in protein kinase Cdelta-null mice. J Clin Invest 2001; 108: 1505–1512.
Sanlioglu AD, Dirice E, Aydin C, Erin N, Koksoy S, Sanlioglu S . Surface TRAIL decoy receptor-4 expression is correlated with TRAIL resistance in MCF7 breast cancer cells. BMC Cancer 2005; 5: 54.
Yagita H, Takeda K, Hayakawa Y, Smyth MJ, Okumura K . TRAIL and its receptors as targets for cancer therapy. Cancer Sci 2004; 95: 777–783.
Kreuz S, Siegmund D, Scheurich P, Wajant H . NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol 2001; 21: 3964–3973.
Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M et al. Inhibition of JNK activation through NF-kappaB target genes. Nature 2001; 414: 313–317.
Chan SW, Hegyi L, Scott S, Cary NR, Weissberg PL, Bennett MR . Sensitivity to Fas-mediated apoptosis is determined below receptor level in human vascular smooth muscle cells. Circ Res 2000; 86: 1038–1046.
Flohe L, Brigelius-Flohe R, Saliou C, Traber MG, Packer L . Redox regulation of NF-kappa B activation. Free Radic Biol Med 1997; 22: 1115–1126.
Nishi T, Shimizu N, Hiramoto M, Sato I, Yamaguchi Y, Hasegawa M et al. Spatial redox regulation of a critical cysteine residue of NF-kappa B in vivo. J Biol Chem 2002; 277: 44548–44556.
Lyle AN, Griendling KK . Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology (Bethesda) 2006; 21: 269–280.
Bowie A, O’Neill LA . Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol 2000; 59: 13–23.
Fontayne A, Dang PM, Gougerot-Pocidalo MA, El-Benna J . Phosphorylation of p47phox sites by PKC alpha, beta II, delta, and zeta: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry 2002; 41: 7743–7750.
Kikkawa U, Matsuzaki H, Yamamoto T . Protein kinase C delta (PKC delta): activation mechanisms and functions. J Biochem 2002; 132: 831–839.
Park HS, Chun JN, Jung HY, Choi C, Bae YS . Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc Res 2006; 72: 447–455.
Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V . Role of proteolytic activation of protein kinase Cdelta in oxidative stress-induced apoptosis. Antioxid Redox Signal 2003; 5: 609–620.
Sumimoto H, Miyano K, Takeya R . Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem Biophys Res Commun 2005; 338: 677–686.
Choi K, Ryu SW, Song S, Choi H, Kang SW, Choi C . Caspase-dependent generation of reactive oxygen species in human astrocytoma cells contributes to resistance to TRAIL-mediated apoptosis. Cell Death Differ 2010; 17: 833–845.
Usui M, Egashira K, Ohtani K, Kataoka C, Ishibashi M, Hiasa K et al. Anti-monocyte chemoattractant protein-1 gene therapy inhibits restenotic changes (neointimal hyperplasia) after balloon injury in rats and monkeys. FASEB J 2002; 16: 1838–1840.
Acknowledgements
This work was supported by the Mid-Career Researcher Program through a National Research Foundation grant funded by the Korean government MEST (no. 2009-0080280 to CC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by P Salomoni
Supplementary Information accompanies the paper on Cell Death and Disease website
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Song, S., Choi, K., Ryu, SW. et al. TRAIL promotes caspase-dependent pro-inflammatory responses via PKCδ activation by vascular smooth muscle cells. Cell Death Dis 2, e223 (2011). https://doi.org/10.1038/cddis.2011.103
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2011.103
Keywords
This article is cited by
-
Regulation of p73 activity by post-translational modifications
Cell Death & Disease (2012)