Abstract
Tropheryma whipplei, the etiological agent of Whipple's disease, is an intracellular bacterium that infects macrophages. We previously showed that infection of macrophages results in M2 polarization associated with induction of apoptosis and interleukin (IL)-16 secretion. In patients with Whipple's disease, circulating levels of apoptotic markers and IL-16 are increased and correlate with the activity of the disease. To gain insight into the understanding of the pathophysiology of this rare disease, we examined the molecular pathways involved in T. whipplei-induced apoptosis of human macrophages. Our data showed that apoptosis induction depended on bacterial viability and inhibition of bacterial protein synthesis reduced the apoptotic program elicited by T. whipplei. Induction of apoptosis was also associated with a massive degradation of both pro- and anti-apoptotic mediators. Caspase-specific inhibition experiments revealed that initiator caspases 8 and 10 were required for apoptosis, in contrast to caspases 2 and 9, in spite of cytochrome-c release from mitochondria. Finally, the effector caspases 3 and 6 were mandatory for apoptosis induction. Collectively, these data suggest that T. whipplei induces apoptosis through the extrinsic pathway and that, beside M2 polarization of macrophages, apoptosis induction contributes to bacterial replication and represents a virulence trait of this intracellular pathogen.
Similar content being viewed by others
Main
Whipple's disease is a rare systemic chronic disease caused by the intracellular pathogen Tropheryma whipplei. This rod-shaped microorganism is a Gram-positive bacterium, classified in the Actinobacteria phylum. Classical symptoms of Whipple's disease associate diarrhea, weight loss, abdominal pain, arthralgia, fever and lymphadenopathy.1 The most severe manifestation of Whipple's disease involves the central nervous system, with disturbance of cognitive functions. Diagnosis of Whipple's disease is usually carried out after Periodic-acid Schiff staining of biological samples and PCR.1 However, it has been shown that T. whipplei may be detected in healthy individuals without any clinical signs of Whipple's disease.2 Immunopathology of Whipple's disease starts to be elucidated. We previously showed that macrophages from patients with Whipple's disease present an alternatively activated phenotype characterized by the expression of arginase, CCL18, cathepsins, scavenger receptor, interleukin (IL)-10 and lipid metabolites.3 In addition, T. whipplei replicates in human macrophages through an IL-16-dependent mechanism, whereas bacteria are eliminated in monocytes.4 Indeed, the production of IL-16 by macrophages in response to T. whipplei is critical for bacterial replication because blocking IL-16 results in bacterial clearance. Conversely, addition of IL-16 to monocytes allows the bacteria to replicate at levels comparable to those observed in macrophages.4 Finally, T. whipplei induces macrophage apoptosis.4 These results were further strengthened by the fact that, in patients, circulating levels of IL-16 and apoptosis markers correlate with the severity of the disease.5
Apoptosis or programmed cell death is a physiological process critical for the maintenance of the immune system. Two pathways govern apoptosis induction, namely the intrinsic and the extrinsic pathways. The intrinsic pathway is initiated from cellular stress signals and involves activation of Bcl-2-like pro-apoptotic proteins of the Bax group (Bax and Bak), which oligomerize and permeabilize the mitochondrial membrane, resulting in cytochrome-c release and initiator caspase activation through apoptosome assembly. Activation of initiator caspases (caspases 2, 8, 9 and 10) induces an expanding cascade that ultimately leads to activation of effector caspases (caspases 3, 6 and 7), which initiate cleavage of specific cellular substrates and thus apoptosis.6 The extrinsic pathway of apoptosis is triggered after binding of a pro-apoptotic ligand to death receptors, which induces receptor clustering and recruitment of adapter proteins that directly activate initiator caspases, thereby converging to the intrinsic pathway.6
Apoptosis can promote efficient pathogen clearance because the death of the host cell is generally associated with the death of the infecting agent. However, several microorganisms have evolved strategies to modulate apoptotic response in the course of infection. Indeed, some of them, such as Rickettsia rickettsii or Chlamydia spp., prevent cell death to replicate intensively within host cells,7, 8 while others, such as Yersinia pestis or Bacillus anthracis, induce apoptosis to favor bacterial spreading.9, 10 In this report, we aimed to examine the molecular pathway induced by T. whipplei to promote macrophage apoptosis. Our results showed that T. whipplei-induced macrophage apoptosis involved the repression of numerous anti-apoptotic mediators, cytochrome-c release from mitochondria, and caspase 8/10 and 3/6 activation, leading to IL-16 production and favoring bacterial replication.
Results
T. whipplei induces apoptosis of human MDM
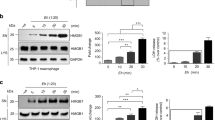
To evaluate the effects of T. whipplei on monocyte-derived macrophage (MDM) apoptosis, cells were infected for 4 h with T. whipplei, washed to discard unbound bacteria and incubated for 24 and 48 h before Annexin V staining. T. whipplei induced MDM apoptosis in a time-dependent manner (Figure 1a). Indeed, at 24 h, 11.5±4.5% of infected MDM were apoptotic, and this percentage increased to 20.1±3.9% for MDM incubated for 48 h, versus 3.8±1.6 and 3.9±1.0% for uninfected MDM incubated for 24 and 48 h, respectively. This result is similar to findings previously observed.4 Interestingly, heat-killed bacteria did not induce significant MDM apoptosis (8.5±1.5%). Transmission electron microscopy (TEM) observation of MDM incubated for 48 h after infection confirmed annexin V findings. MDM showed characteristic features of apoptosis, including vacuolation and chromatin condensation (Figure 1b). To examine whether de novo T. whipplei protein synthesis was required for inducing apoptosis, MDM were infected with T. whipplei in the presence of the bacterial protein synthesis inhibitor, chloramphenicol. We found that chloramphenicol dramatically reduced T. whipplei-induced apoptosis, while it had no effect on UV-induced apoptosis of MDM (Figure 1c). To avoid any bias resulting from the effects of chloramphenicol on mitochondrial protein synthesis, we used another bacterial protein synthesis inhibitor, streptomycin. As observed with chloramphenicol, streptomycin dramatically reduced apoptosis induced by T. whipplei (data not shown). Collectively, these results show that T. whipplei induces macrophage apoptosis and suggest that de novo protein synthesis is required for apoptosis induction.
T. whipplei induces apoptosis of human MDM. (a) MDM were infected with live or heat-killed (HK) T. whipplei (MOI 50 : 1) for 4 h, washed and incubated for 24 and 48 h. Cells were then washed and stained with annexin V-FITC and PI and analyzed by flow cytometry. The data are the mean±S.D. of three independent experiments. (b) Uninfected (left) and T. whipplei-infected (right) MDM were observed by TEM after 48 h of incubation. (c) T. whipplei-infected MDM were incubated for 48 h in the presence or not of chloramphenicol (Cm, 20 μg/ml). As a control, MDM were exposed to UV light. Apoptosis was determined by TUNEL staining and quantified by examining 3–5 fields per condition (∼100 cells per field). The percentage of TUNEL-positive cells was calculated as the ratio between TUNEL-positive and DAPI-stained nuclei × 100. *P<0.05, Mann–Whitney's U-test
T. whipplei infection results in a massive degradation of apoptosis-related proteins
To better characterize molecular events leading to MDM apoptosis after infection with T. whipplei, we conducted an apoptosis-related protein antibody array analysis of MDM infected or not with T. whipplei. Nineteen proteins, either pro- or anti-apoptotic, were significantly modulated (Figure 2). Among proteins known to mediate apoptosis, only levels of cleaved caspase 3 were increased (P<0.05) whereas Bad, Bax, FADD, Fas/TNFSF6 and p27/Kip1 were either unchanged or repressed (Figure 2a). Similarly, levels of most of anti-apoptotic proteins were reduced after infection. Members of the inhibitor apoptosis family (IAPs) such as XIAP and livin were inhibited. Members of the heat shock proteins (HSPs), Hsp27 and Hsp70, as well as detoxifying enzymes such as catalase, heme oxygenase 1 and 2 (HO-1 and HO-2), and paraoxonase 2 (PON2) were also significantly reduced by T. whipplei (Figure 2b). Strikingly, one anti-apoptotic protein, namely p21/CIP1/CDNK1A, was strongly increased on infection. These findings suggest that T. whipplei infection results in a marked modulation of apoptosis-related proteins.
T. whipplei modulates the cellular content of pro- and anti-apoptotic mediators. MDM were infected with T. whipplei (MOI 50 : 1) for 4 h, washed and incubated for 24 h. Cell lysates were applied on a human apoptosis protein array. The average density of duplicate spots representing each pro-apoptotic (a) and anti-apoptotic (b) proteins was expressed in arbitrary units (AU). Significant changes in protein expression after T. whipplei infection were determined by two-way ANOVA (*P<0.05; ***P<0.001)
T. whipplei induces mitochondrial transmembrane potential disturbance
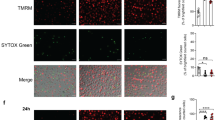
To further examine the apoptotic effect of T. whipplei infection, we monitored the decrease in the mitochondrial transmembrane potential (Δψm) and the release of cytochrome-c, which are associated with the induction of apoptosis.11 Changes in Δψm were analyzed after incubation of MDM with the cationic dye DiOC6[3] by flow cytometry. As expected, mitochondria from uninfected MDM retained DiOC6[3] and a marked decrease of fluorescence was observed when cells were treated with staurosporine. When cells were infected with T. whipplei and incubated for 24 and 48 h, the fluorescence decreased, suggesting a leakage of DiOC6[3] from mitochondria (Figure 3a). We also monitored cytochrome-c release by immunoblot analysis. Cytochrome-c release into the cytosolic fraction was detected after treatment of MDM with staurosporine. For cells infected with T. whipplei, cytochrome-c release started to be observed after 24 h of incubation and was evident at 48 h (Figure 3b), thereby confirming the decrease of Δψm.
T. whipplei triggers Δψm disturbance and caspase activation. MDM were infected with T. whipplei (MOI 50 : 1) for 4 h, washed and incubated for additional periods. As a control, MDM were treated with staurosporine (Staur) for 6 h. (a) Cells were incubated with DiOC6[3] and fluorescence was monitored by flow cytometry. The results are representative of two independent experiments (gray line: uninfected cells; black line: staurosporine-treated cells; blue and red line: MDM incubated 24 and 48 h after infection, respectively). (b) MDM lysates were analyzed by immunoblotting for cytochrome-c, caspase 8, caspase 9 or α-tubulin as loading control. One representative experiment is shown (n=2). (c) Caspase 3 activity was determined by immunofluorescence using active caspase 3-specific antibodies. (d) MDM were infected with T. whipplei (MOI 50 : 1) for 4 h, washed and incubated for 24 and 48 h in the presence of different caspase inhibitors. Apoptosis was determined by TUNEL staining and quantified by examining 3–5 fields per condition. The percentage of TUNEL-positive cells was calculated as the ratio between TUNEL-positive and DAPI-stained nuclei × 100. (e) MDM lysates were analyzed by immunoblotting for IL-16 and α-tubulin as loading control. Arrows represent the cleaved forms of IL-16. One representative experiment is shown (n=2). (f) MDM supernatants were assessed for IL-16 by ELISA. The data are the mean±S.D. of three experiments. *P<0.05, Mann–Whitney's U-test
T. whipplei-induced apoptosis involves caspase activity
To determine whether caspases are involved in T. whipplei-induced apoptosis of MDM, we examined the initiator caspases, caspase 8 and caspase 9, and the executioner caspase, caspase 3. As determined by western blot, we found that the expression of caspase 8 was induced within 24 h and increased further at 48 h, but we were not able to observe caspase 9 expression (Figure 3b). As expected for its role in the initiation of apoptosis, caspase 8 induction occurred early before cell death because it was detectable at 6 h while MDM apoptosis became significantly increased at 24 and 48 h after infection (Supplementary Figure 1). The executioner caspase 3 was clearly activated 48 h after infection of MDM with T. whipplei, as shown by immunofluorescence (Figure 3c). To further analyze the role of caspases in T. whipplei-induced apoptosis, we performed caspase inhibition experiments using the pan caspase inhibitor (Z-VAD-FMK), the initiator caspase 2-, 8-, 9- and 10-specific inhibitors, Z-VDVAD-FMK, Z-IETD-FMK, Z-LEHD-FMK and Z-AEVD-FMK, respectively, and the effector caspase 3- and 6-specific inhibitors, Z-DEVD-FMK and Z-VEID-FMK, respectively (Figure 3d). Treatment of MDM with the pan caspase inhibitor completely abolished T. whipplei-induced apoptosis. In addition, we found that T. whipplei activated specific initiator caspases. Indeed, inhibition of caspase 8 and caspase 10 resulted in a significant reduction of the percentage of apoptotic cells whereas inhibition of caspase 2 and caspase 9 had no effect (Figure 3d). In contrast, we found that the executioner caspases were necessary for T. whipplei-induced apoptosis as the inhibition of both caspase 3 and caspase 6 significantly prevented apoptosis (Figure 3d). These findings suggest that T. whipplei-induced apoptosis requires activities of the initiator caspase 8/10 and the executioner caspase 3/6.
Activated caspase 3 is known to initiate the cleavage of specific cellular substrates.6 We thus analyzed the cleavage of one of its substrates that has also been involved during Whipple's disease, pro-IL-16. Immunoblot analysis showed that infection with T. whipplei caused the cleavage of pro-IL-16 in a time-dependent manner to yield the biologically active IL-16. Pro-IL-16 cleavage products were evidenced at 24 h and their levels increased at 48 h (Figure 3e, arrows). As a result, biologically active IL-16 was in turn secreted by MDM (Figure 3f). Collectively, these results show that apoptosis induced by T. whipplei involves caspase activation.
Apoptosis induction favors T. whipplei replication in macrophages
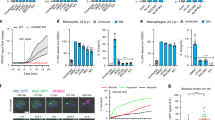
Finally, to determine whether apoptosis induction contributes to bacterial replication, we prevented T. whipplei-induced apoptosis and monitored bacterial replication by real-time quantitative PCR. Single inhibition of caspases 8 and 10 resulted in a 64.7 and 67.8% apoptosis inhibition, respectively (Figure 3d). We wondered if double inhibition of caspases 8 and 10 would inhibit even more the cell death induced by T. whipplei. Simultaneous treatment of MDM with both caspase 8 and caspase 10 inhibitors reduced T. whipplei-induced apoptosis by 67.6% reduction, which was similar to the reduction observed with single inhibitors (Figure 4a). These results suggest that both caspase 8 and caspase 10 are required for apoptosis induction after T. whipplei infection and that one might act upstream of the other. Next, we followed bacterial replication over a 12-day period. In the absence of inhibitors, T. whipplei replicates in MDM as previously described.4 After 4 h (uptake), around 3000 T. whipplei DNA copies were detected. In the first 3 days, bacterial DNA copy number decreased and started to increase after 6 days to reach around 30 000 copies after 12 days. In contrast, in the presence of caspase 8 and 10 inhibitors, replication of T. whipplei was significantly reduced in MDM, as around 10 000 DNA copies were detected at day 12 (Figure 4b). Overall, these results suggest that apoptosis induction contributes to macrophage permissivity toward T. whipplei.
Apoptosis induction is required for T. whipplei replication. (a) MDM were infected with T. whipplei (MOI 50 : 1) for 4 h, washed and incubated for 48 h in the presence of caspase 8 inhibitor, caspase 10 inhibitor, or both. Apoptosis was determined by TUNEL staining and quantified by examining 3–5 fields per condition. The percentage of TUNEL-positive cells was calculated as the ratio between TUNEL-positive and DAPI-stained nuclei × 100 (control: uninfected cells after incubation for 48 h). (b) MDM were infected with T. whipplei (MOI 50 : 1) for 4 h (uptake), washed and incubated for different periods in the presence of both caspase 8 and 10 inhibitors. Levels of bacterial DNA copy number were determined by qPCR (n=3). *P<0.05, Mann–Whitney's U-test
Discussion
One of the most striking characteristics of Whipple's disease is the accumulation of T. whipplei organisms in macrophages in the duodenal mucosa, suggesting that macrophages are the target of an effective immune evasion strategy of T. whipplei.3, 12 Macrophages are long-lived cells that have a critical role in immune responses and resolution of inflammation. We previously showed that T. whipplei induces macrophage apoptosis4 and that apoptotic markers were increased in patients with active Whipple's disease.5 In addition, apoptosis induction is associated with the M2 polarization of macrophages, thereby constituting a favorable niche for bacterial replication.3 In this report, we aimed at defining the molecular events leading to macrophage apoptosis after infection with T. whipplei. We found that apoptosis induction required bacterial viability and de novo protein synthesis, as cells infected with heat-killed bacteria or treated with chloramphenicol or streptomycin did not undergo apoptosis. Modulation of apoptosis by bacterial proteins synthetized de novo has already been observed in other models of infection, such as Coxiella burnetii13 or Mycobacterium tuberculosis.14
Induction of apoptosis was associated with a disturbance of pro- and anti-apoptotic mediators. A majority of them were repressed after infection except cleaved-caspase 3 and p21/CIP1/CDNK1A, which were markedly induced. The increased activity of caspase 3 can be linked to the diminution of cellular IAP levels (mainly XIAP, but also livin). Indeed, IAPs are potent inhibitors of caspases, and their repression might change the balance toward apoptosis. Induction of apoptosis may also be linked to the significant reduction of detoxifying/repair proteins, as well as HO-1 and HO-2. HO-1 is an inducible HSP (Hsp32), which is upregulated in stress conditions and reflects oxidative stress both in vivo and in vitro.15 HO-1 has been shown to inhibit apoptosis of various cell types, including monocytes/macrophages.16 HO-2 is constitutively expressed and also appears as a critical regulator of apoptosis by controlling levels of extracellular superoxide dismutase and apoptotic signaling kinase-1.17 We also found that cellular levels of chaperones (Hsp27 and Hsp70) and repair enzymes, such as PON2 or detoxifying enzymes, such as catalase, were diminished after T. whipplei infection. The deregulation of these proteins might severely impair detoxification of reactive oxygen species (ROS) and thus contribute to apoptosis induction. This hypothesis is strengthened by the fact that other redox-related molecules, such as thioredoxin and glutaredoxin, have been shown to be repressed in macrophages after infection by T. whipplei.4
MDM apoptosis induced by T. whipplei was associated with a decrease of Δψm, and the release of cytochrome-c in the cytosol. M. tuberculosis-infected macrophages also undergo apoptosis, and virulence of M. tuberculosis is correlated with mitochondrial cytochrome-c release.18 In the case of T. whipplei, the decrease of anti-oxidant mechanisms (see above) may lead to ROS accumulation, resulting in a Δψm decrease and cytochrome-c release from the mitochondrial membrane.19 Cytochrome-c release in the cytosol in turn activates caspase 9 after binding to Apaf-1 through formation of the apoptosome.20 Nevertheless, we were not able to detect caspase 9 activation, or modulation of caspase 9 steady-state protein levels after T. whipplei infection. In addition, its specific inhibition had no apparent effect on T. whipplei-induced apoptosis whereas caspase 3 appeared critical. As cytochrome-c can inhibit caspase 9 activity in vitro,21 it is therefore possible that caspase 3 acts upstream of mitochondria, resulting in Δψm decrease as it has been already described.22
We then treated MDM with specific inhibitors of initiator caspases. Caspase 2 and caspase 9, which are primarily activated through the intrinsic pathway,23 were not involved in MDM apoptosis induced by T. whipplei, as their respective inhibition had no effect. In contrast, the initiator caspases 8 and 10 were required for apoptosis induction. Caspase 8 is primarily activated through the extrinsic apoptosis pathway: stimulation of death receptors of the tumor necrosis factor (TNF) receptor superfamily results in the activation of the initiator caspase 8. Recently, we found in murine macrophages that T. whipplei induces after 6 h the overexpression of the genes encoding Fas, TRAIL, but also type I IFN, which is involved in apoptosis induction.24 It is tempting to speculate that caspase 8 was activated after stimulation of death receptors. However, we found here that the steady-state protein levels of Fas, TRAIL R2 but also FADD were decreased 24 h after infection. These discrepancies between transcriptional and protein data might arise from a post-transcriptional regulation of mRNA, or alternatively, Fas, TRAIL R2 and FADD might readily be synthesized, and degraded later on, when apoptosis is engaged. In addition, type I IFNs have been clearly shown to induce apoptosis and activate the caspase 8 signaling and the caspase cascade.25 Caspase 10, which is very closely related to caspase 8, is also recruited after engagement of death receptors and acts upstream of caspase 3.26 Collectively, these data strongly suggest that T. whipplei-induced apoptosis follows the extrinsic pathway.
We also showed that the effector caspases 3 and 6 were involved and required for apoptosis induction. It has been shown that activation of caspase 3 results in the activation of caspase 6 and that, at least in vitro, caspase 6 can process pro-caspase 3, thus resulting in an amplification cycle.26 In addition, pro-IL-16, one of the caspase 3 targets,27 was cleaved, resulting in the secretion of bioactive IL-16 by MDM. Thus, during T. whipplei infection, apoptosis and IL-16 are intimately associated. In patients with active Whipple's disease, circulating apoptotic markers and IL-16 levels are correlated.5 Successful treatment of patients results in the decrease of circulating apoptotic markers and IL-16 levels, while they are increased when patients relapse.5 Moreover, macrophages from these patients are characterized by M2 polarization.3 Interestingly, we found that one of the proteins that was upregulated in response to T. whipplei was p21/CIP1/CDNK1A, also known as p21Cip1/WAF1. p21Cip1/WAF1 is a cyclin-dependent kinase (CDK) inhibitor, which belongs to the Cip/Kip family.28 Initially described as promoting cell cycle arrest, p21Cip1/WAF1 has also been shown to be a critical regulator of apoptosis and differentiation.29 p21Cip1/WAF1 protects cells against apoptosis through its CDK inhibitory activity, but also by controlling activity of transcription factors such as E2F1, c-Myc or STAT3. p21 can also bind directly to pro-caspase-3 or to JNK kinases, thereby inhibiting their activation.28 However, p21Cip1/WAF1 has also been reported to have a pro-apoptotic role, depending on the cell type and apoptotic stimulus.30 In those cases, expression of p21Cip1/WAF1 appears STAT1-dependent.31 It is interesting to note that T. whipplei induces STAT1 activation in an type I IFN-dependent mechanism24 and that type I IFN activates the p21 gene promoter and induces p21Cip1/WAF1 expression.32 Beyond these cell cycle/apoptosis functions, recent findings have shown a novel role for p21 as a negative regulator of macrophage activation, independently of cell cycle or apoptosis.33, 34 Indeed, mice deficient for p21 show increased susceptibility to endotoxic shock, associated with increased circulating levels of IL-1β.34 In LPS-stimulated p21-deficient macrophages, activity of the transcription factor NF-κB is increased, resulting in the over-production of pro-inflammatory mediators, such as TNF and IL-1β.33 We previously found that macrophage responses to T. whipplei are characterized by the absence of pro-inflammatory mediators and M2 polarization.12, 24 Hence, we can hypothesize that T. whipplei modulates macrophage activation, at least by increasing cellular p21 levels.
Finally, we showed here that apoptosis induction was linked to T. whipplei replication: prevention of apoptosis by inhibiting the initiator caspases 8 and 10 resulted in a marked reduction of bacterial replication. Clearance of apoptosis cells by professional phagocytes such as macrophages results in a powerful anti-inflammatory and immunosuppressive effect.35 In another infection model, it has been shown that phagocytosis of apoptotic cells by macrophages promotes their M2 polarization, allowing intense C. burnetii intracellular replication.36 As a result, it is probable that, after apoptosis induction by T. whipplei, ingestion of apoptotic cells by surrounding macrophages triggers a potent anti-inflammatory response, favoring bacterial replication. Therefore, apoptosis induction and p21 overexpression might act in concert to subvert macrophage responses, at least by modulating their activation and polarization.
In conclusion, we show that T. whipplei induces apoptosis in macrophages through a caspase 8- and caspase 3-dependent pathway, associated with IL-16 secretion and the loss of mitochondrial membrane potential. Our findings provide promising insight into the host cell responses during Whipple's disease and offer molecular bases in the understanding of the pathophysiology of Whipple's disease.
Materials and Methods
Cell culture, bacterial infection and reagents
Monocytes were isolated from buffy coats obtained at the French blood bank (Etablissement Français du Sang) by ficoll gradient centrifugation and CD14 selection with MACS magnetic microbeads (Miltenyi Biotec, Paris, France). Isolated monocytes were cultivated in RPMI 1640 medium containing 10% heat-inactivated human AB serum and 2 mM glutamine (Sigma, Saint Quentin Fallavier, France). After the first 3 days, medium was replaced by RPMI 1640 containing 10% fetal calf serum (FCS) and 2 mM glutamine. After 7 days of culture, more than 85% of the resulting MDMs expressed CD68 and were used for further experiments.
The strain Twist–Marseille of T. whipplei (CNCM I-2202) was cultured in HEL cells and purified as described previously.4 Bacteria were counted by indirect immunofluorescence and their viability was assessed using the LIVE/DEAD BacLight bacterial viability kit (Invitrogen, Cergy Pontoise, France). Heat-killed T. whipplei was prepared by heating at 80 °C for 1 h. T. whipplei organisms (MOI 50 : 1) were added to MDM for 4 h, washed to remove free bacteria and incubated for different times in RPMI 1640 containing 10% FCS and 2 mM glutamine.
In some experiments, apoptosis was induced using staurosporine (Sigma) at 500 nM for 4–6 h. As needed, 20 μg/ml chloramphenicol or 10 μg/ml streptomycin (MIC=1 μg/ml for T. whipplei in MRC5 cells37) was added to MDM cultures. For UV irradiation experiments, seeded MDM were placed in a tissue culture hood at a distance of 60 cm from the UV-C light source as described previously.38 For caspase inhibition, MDM were incubated with 20 μM of the pan caspase inhibitor (Z-VAD-FMK), caspase 2 inhibitor (Z-VDVAD-FMK), caspase 8 inhibitor (Z-IETD-FMK), caspase 9 inhibitor (Z-LEHD-FMK), caspase 10 inhibitor (Z-AEVD-FMK), caspase 3 inhibitor (Z-DEVD-FMK) or caspase 6 inhibitor (Z-VEID-FMK) (all from R&D Systems, Lille, France) for 1 h before infection and maintained during the course of the experiments.
Annexin V staining
Infected MDM, seeded on six-well plates at 106 cells per well, were incubated for 24 and 48 h, scrapped and then washed with PBS, before staining with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) according to the manufacturer's protocol (AbCys). The percentage of apoptotic cells (annexin+/PI−) was determined by flow cytometry using a FACSCalibur (Beckman Dickinson, Le Pont de Claix, France), and the data were analyzed with Cyflogic software (CyFlo Ltd, Turku, Finland).
Human apoptosis protein array
To compare the levels of apoptosis-related proteins before and after T. whipplei infection, a human apoptosis protein array (R&D Systems) was used according to manufacturer's instructions. Briefly, MDM were infected with T. whipplei for 4 h, washed and incubated for 24 h. Protein lysates (500 μg) were incubated onto an array membrane at 4 °C overnight, washed three times for 5 min, and then incubated with a horseradish peroxidase (HRP)-linked secondary antibody at a dilution of 1 : 2000. After washing, blotting dots were visualized by Immobilon Western Chemiluminescent HRP substrate (Millipore, Guyancourt, France). Densitometry of protein dot signals was obtained. Normalized intensities were calculated from each array by subtracting the local background from each spot, and the data were corrected for the protein content of each well. The average density of duplicate spots representing each apoptosis-related protein indicated its relative levels and expressed in arbitrary units. Significant changes in protein expression after T. whipplei infection were determined by two-way ANOVA (P<0.05).
Transmission electron microscopy
MDM were scrapped and fixed in 2.5% glutaraldehyde diluted in 0.1 M cacodylate buffer (pH 7.2) containing 0.1 M sucrose for 1 h at 4 °C. After washing, cells were incubated with 1% osmium tetroxide diluted in 0.1 M cacodylate buffer for 1 h. Dehydration was performed through washes of graded concentrations of acetone (25–100%), and cells were then embedded in Araldite (Sigma-Aldrich). Sections from embedded blocks were post-stained with saturated solution of methanol/uranyl acetate and lead nitrate with sodium citrate in water before examination using a JEOL 1220 electron microscope (JEOL SAS, Croissy-sur-Seine, France).
Terminal transferase deoxytidyl uridine end labeling (TUNEL) staining
Detection of apoptosis by TUNEL was performed using In situ Cell Death Detection Kit, TMR red (Roche, Meylan, France) according to the manufacturer's instructions. After treatment as indicated, cells seeded on glass coverslips were fixed in 4% paraformaldehyde for 15 min, washed in PBS and permeabilized with 0.1% Triton-X100 in 0.1% sodium citrate for 2 min. Cells were then incubated with the TUNEL mixture containing TMR-dUTP and terminal deoxynucleotidyl transferase for 1 h. Cells were washed in PBS and nuclei were stained with DAPI before mounting with Mowiol. Positive controls were carried out by incubating cells with 3 U/ml DNase I prior labeling procedures. Negative controls were performed by incubating cells with label solution (without terminal deoxynucleotidyl transferase). Apoptosis was quantified as follows. Coverslips were examined in fluorescence mode with a Leica microscope (Leica Microsystems, Nanterre, France) equipped with a Nikon digital camera (Nikon Instruments, Champigny sur Marne, France) using a 10 × objective lens. Three to five fields per condition (100–300 cells each) were observed. The number of TUNEL-positive and DAPI-stained nuclei was determined and the apoptosis percentage was expressed as the ratio between TUNEL-positive and DAPI-stained nuclei × 100.
Cytochrome-c release
Cytosolic fractions from MDM were prepared as described previously.39 Cell lysates were examined for equal amounts of proteins by the Bradford method using γ globuline as a standard. Samples were loaded onto 10% sodium dodecyl sulfate (SDS) polyacrylamide gels, electrophoresed and transferred onto nitrocellulose membranes (GE Healthcare, Saclay, France). The membranes were blocked in PBS with 0.05% Tween 20 (PBST) supplemented with 3% powdered milk and then incubated with anti-cytochrome-c Ab (BD Pharmingen, Le Pont de Claix, France) or α-tubulin Ab (Cell Signaling, Danvers, MA, USA) as a loading control. The blots were washed with PBST and incubated with a secondary Ab, either HRP-conjugated anti-rabbit or anti-mouse immunoglobulin (Pierce, Rockford, IL, USA) in PBST plus 3% powdered milk. The bound Abs were detected using Immobilon Western Chemiluminescent HRP substrate (Millipore).
Analysis of transmembrane mitochondrial potential (Δψm)
The analysis of Δψm was performed using the dye DiOC6[3]. This dye strongly labels mitochondria and a decrease in Δψm in apoptotic cells is associated with a reduction of DiOC6[3] uptake after flow cytometry analysis.40 In brief, MDM were either treated with staurosporine for 6 h, infected with T. whipplei for 4 h and incubated for additional times, or left untreated. Cells were then incubated with the cationic at 20 nM for 30 min at 37 °C and analyzed by flow cytometry.
Western blot analysis
At designated times after infection, MDM were washed with ice-cold PBS. Cells were then scrapped in ice-cold RIPA buffer (20 mM Tris-HCl, 200 mM NaCl, 1 mM EDTA, 1% Triton -X100, pH 7.5) containing protease inhibitor (Complete, Roche) and phosphatase inhibitor (Phosphostop, Roche) cocktails. The cell lysates were cleared by centrifugation at 14 000 r.p.m. for 15 min at 4 °C and stored at −80 °C. Samples were loaded onto 10% SDS polyacrylamide gels, electrophoresed and transferred onto nitrocellulose membranes (Amersham) before probing with Abs against caspase 8, caspase 9 (BD Pharmingen) or α-tubulin, as indicated by the manufacturer. Bound Abs were detected as described above.
Caspase 3 activation
Direct caspase 3 activation was determined by indirect immunofluorescence as followed: 48 h after infection, MDM were fixed in 3% paraformaldehyde and permeabilized with 0.1% Triton -X100. Immunofluorescence labeling was performed according to standard procedures. Briefly, MDM were incubated with rabbit anti-active caspase 3 polyclonal Ab (Biovision, Mountain View, CA, USA, 1 : 100 dilution) for 30 min. Secondary anti-rabbit 488-Alexa Abs were purchased from Invitrogen and used at a 1 : 500 dilution. Coverslips were mounted with Mowiol and examined in fluorescence mode with a Leica microscope equipped with a Nikon digital camera using a 100 × objective lens. Caspase 3 activation was also monitored through the cleavage of one of its substrates, IL-16.27 IL-16 cleavage was determined by western blot as described above by incubating the membranes with mouse monoclonal anti-IL-16 (R&D Systems). Alternatively, IL-16 protein release was assessed in culture supernatant by ELISA (R&D Systems), according to the manufacturer's instructions.
Real-time quantitative PCR (qPCR)
T. whipplei organisms (MOI 50 : 1) were added to MDM for 4 h, washed to remove free bacteria and incubated for 12 days in RPMI 1640 containing 10% FCS and 2 mM glutamine. Every 3 days, MDM were collected and DNA was extracted using the QIAamp DNA MiniKit (Qiagen, Courtaboeuf, France). PCR was performed using the LightCycler-FastStart DNA Master SYBR Green system (Roche), as previously described.4
Statistical analysis
All experiments were performed at least two or three times. Error bars represent S.D. *P<0.05, Mann–Whitney's U-test.
Abbreviations
- IL:
-
interleukin
- MDM:
-
monocyte-derived macrophage
- TUNEL:
-
Terminal transferase deoxytidyl uridine end labeling
- IAP:
-
inhibitor of apoptosis
- HSP:
-
heat shock protein
- ΔΨm:
-
mitochondrial transmembrane potential
- MOI:
-
multiplicity of infection
- TNF:
-
tumor necrosis factor
- ROS:
-
reactive oxygen species
- CDK:
-
cyclin-dependent kinase
- qPCR:
-
real-time quantitative PCR
- TEM:
-
transmission electron microscopy
- DMSO:
-
dimethyl sulfoxide
- PBST:
-
PBS-Tween
- FCS:
-
fetal calf serum
- HRP:
-
horseradish peroxidase
- SDS:
-
sodium dodecyl sulfate
References
Schneider T, Moos V, Loddenkemper C, Marth T, Fenollar F, Raoult D . Whipple's disease: new aspects of pathogenesis and treatment. Lancet Infect Dis 2008; 8: 179–190.
Rolain JM, Fenollar F, Raoult D . False positive PCR detection of Tropheryma whipplei in the saliva of healthy people. BMC Microbiol 2007; 7: 48.
Desnues B, Lepidi H, Raoult D, Mege JL . Whipple disease: intestinal infiltrating cells exhibit a transcriptional pattern of M2/alternatively activated macrophages. J Infect Dis 2005; 192: 1642–1646.
Desnues B, Raoult D, Mege JL . IL-16 is critical for Tropheryma whipplei replication in Whipple's disease. J Immunol 2005; 175: 4575–4582.
Benoit M, Fenollar F, Raoult D, Mege JL . Increased levels of circulating IL-16 and apoptosis markers are related to the activity of Whipple's disease. PloS One 2007; 2: e494.
Kurokawa M, Kornbluth S . Caspases and kinases in a death grip. Cell 2009; 138: 838–854.
Clifton DR, Goss RA, Sahni SK, van Antwerp D, Baggs RB, Marder VJ et al. NF-kappa B-dependent inhibition of apoptosis is essential for host cell survival during Rickettsia rickettsii infection. Proc Natl Acad Sci USA 1998; 95: 4646–4651.
Fan T, Lu H, Hu H, Shi L, McClarty GA, Nance DM et al. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med 1998; 187: 487–496.
Hanna PC, Acosta D, Collier RJ . On the role of macrophages in anthrax. Proc Natl Acad Sci USA 1993; 90: 10198–10201.
Riedl SJ, Salvesen GS . The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol 2007; 8: 405–413.
Matsuyama S, Reed JC . Mitochondria-dependent apoptosis and cellular pH regulation. Cell Death Differ 2000; 7: 1155–1165.
Desnues B, Ihrig M, Raoult D, Mege JL . Whipple's disease: a macrophage disease. Clin Vaccine Immunol 2006; 13: 170–178.
Voth DE, Howe D, Heinzen RA . Coxiella burnetii inhibits apoptosis in human THP-1 cells and monkey primary alveolar macrophages. Infect Immun 2007; 75: 4263–4271.
Loeuillet C, Martinon F, Perez C, Munoz M, Thome M, Meylan PR . Mycobacterium tuberculosis subverts innate immunity to evade specific effectors. J Immunol 2006; 177: 6245–6255.
Maines MD . The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol 1997; 37: 517–554.
Lang D, Reuter S, Buzescu T, August C, Heidenreich S . Heme-induced heme oxygenase-1 (HO-1) in human monocytes inhibits apoptosis despite caspase-3 up-regulation. Int Immunol 2005; 17: 155–165.
Turkseven S, Drummond G, Rezzani R, Rodella L, Quan S, Ikehara S et al. Impact of silencing HO-2 on EC-SOD and the mitochondrial signaling pathway. J Cell Biochem 2007; 100: 815–823.
Abarca-Rojano E, Rosas-Medina P, Zamudio-Cortez P, Mondragon-Flores R, Sanchez-Garcia FJ . Mycobacterium tuberculosis virulence correlates with mitochondrial cytochrome c release in infected macrophages. Scand J Immunol 2003; 58: 419–427.
Chen Q, Crosby M, Almasan A . Redox regulation of apoptosis before and after cytochrome c release. Korean J Biol Sci 2003; 7: 1–9.
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997; 91: 479–489.
Mishra OP, Delivoria-Papadopoulos M . ATP and cytochrome c-dependent inhibition of caspase-9 activity in the cerebral cortex of newborn piglets. Neurosci Lett 2004; 364: 119–123.
Lakhani SA, Masud A, Kuida K, Porter Jr GA, Booth CJ, Mehal WZ et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 2006; 311: 847–851.
Samraj AK, Sohn D, Schulze-Osthoff K, Schmitz I . Loss of caspase-9 reveals its essential role for caspase-2 activation and mitochondrial membrane depolarization. Mol Biol Cell 2007; 18: 84–93.
Al Moussawi K, Ghigo E, Kalinke U, Alexopoulou L, Mege JL, Desnues B . Type I interferon induction is detrimental during infection with the Whipple's disease bacterium, Tropheryma whipplei. PLoS Pathog 2010; 6: e1000722.
Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH et al. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis 2003; 8: 237–249.
Cohen GM . Caspases: the executioners of apoptosis. Biochem J 1997; 326: 1–16.
Zhang Y, Center DM, Wu DM, Cruikshank WW, Yuan J, Andrews DW et al. Processing and activation of pro-interleukin-16 by caspase-3. J Biol Chem 1998; 273: 1144–1149.
Besson A, Dowdy SF, Roberts JM . CDK inhibitors: cell cycle regulators and beyond. Dev Cell 2008; 14: 159–169.
Asada M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumuro K et al. Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. EMBO J 1999; 18: 1223–1234.
Ahmad N, Adhami VM, Afaq F, Feyes DK, Mukhtar H . Resveratrol causes WAF-1/p21-mediated G(1)-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin Cancer Res 2001; 7: 1466–1473.
Agrawal S, Agarwal ML, Chatterjee-Kishore M, Stark GR, Chisolm GM . Stat1-dependent, p53-independent expression of p21(waf1) modulates oxysterol-induced apoptosis. Mol Cell Biol 2002; 22: 1981–1992.
Katayama T, Nakanishi K, Nishihara H, Kamiyama N, Nakagawa T, Kamiyama T et al. Type I interferon prolongs cell cycle progression via p21WAF1/CIP1 induction in human colon cancer cells. Int J Oncol 2007; 31: 613–620.
Trakala M, Arias CF, Garcia MI, Moreno-Ortiz MC, Tsilingiri K, Fernandez PJ et al. Regulation of macrophage activation and septic shock susceptibility via p21(WAF1/CIP1). Eur J Immunol 2009; 39: 810–819.
Scatizzi JC, Mavers M, Hutcheson J, Young B, Shi B, Pope RM et al. The CDK domain of p21 is a suppressor of IL-1beta-mediated inflammation in activated macrophages. Eur J Immunol 2009; 39: 820–825.
Savill J, Dransfield I, Gregory C, Haslett C . A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev 2002; 2: 965–975.
Benoit M, Ghigo E, Capo C, Raoult D, Mege JL . The uptake of apoptotic cells drives Coxiella burnetii replication and macrophage polarization: a model for Q fever endocarditis. PLoS Pathog 2008; 4: e1000066.
Boulos A, Rolain JM, Raoult D . Antibiotic susceptibility of Tropheryma whipplei in MRC5 cells. Antimicrob Agents Chemother 2004; 48: 747–752.
Wang L, Lu L . Pathway-specific effect of caffeine on protection against UV irradiation-induced apoptosis in corneal epithelial cells. Invest Ophthalmol Vis Sci 2007; 48: 652–660.
Luhrmann A, Roy CR . Coxiella burnetii inhibits activation of host cell apoptosis through a mechanism that involves preventing cytochrome c release from mitochondria. Infect Immun 2007; 75: 5282–5289.
Shapiro HM . Membrane potential estimation by flow cytometry. Methods 2000; 21: 271–279.
Acknowledgements
We thank Didier Raoult for assistance and support. Khatoun Al Moussawi is a fellow of the Scientific Cooperation Foundation ‘Infectiopole Sud’. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by M Piacentini
Supplementary Information accompanies the paper on Cell Death and Disease website
Supplementary information
Rights and permissions
This article is licensed under a Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Gorvel, L., Al Moussawi, K., Ghigo, E. et al. Tropheryma whipplei, the Whipple's disease bacillus, induces macrophage apoptosis through the extrinsic pathway. Cell Death Dis 1, e34 (2010). https://doi.org/10.1038/cddis.2010.11
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2010.11
Keywords
This article is cited by
-
Codon usage studies and epitope-based peptide vaccine prediction against Tropheryma whipplei
Journal of Genetic Engineering and Biotechnology (2022)
-
hPNAS-4 inhibits proliferation through S phase arrest and apoptosis: underlying action mechanism in ovarian cancer cells
Apoptosis (2013)
-
Analysis of apoptosis methods recently used in Cancer Research and Cell Death & Disease publications
Cell Death & Disease (2012)
-
Cell death in disease: from 2010 onwards
Cell Death & Disease (2011)