Abstract
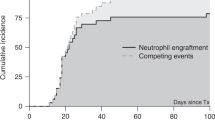
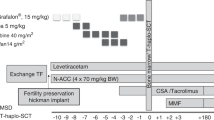
Immune recovery (IR) after haploidentical stem cell transplantation (haplo-SCT) in severe aplastic anemia (SAA) patients remains relatively unknown. In this study, we examined immune cell subset counts and immunoglobulins in 81 SAA patients from day 30 to day 365 after haplo-SCT. Simultaneously, we determined which factors influence IR and analyzed the effects of immune cell subsets on transplant outcomes. We found that: (i) The reconstitution of different immune cell subsets occurred at different rates after haplo-SCT. Monocytes were the first to recover, followed by CD8+ T and CD19+ B cells, and finally CD4+ T cells. (ii) In the multivariate analysis, lower recipient age, female gender, high mononuclear cell counts in the graft and absence of CMV reactivation were associated with improved IR after transplant. (iii) A CD4/CD8 ratio less than 0.567 on day 30 post transplantation was associated with higher overall survival after haplo-SCT in SAA patients. In conclusion, SAA patients showed a faster recovery of monocytes and CD8+ T cells after haplo-SCT, whereas the recovery of the CD4+ T-cell subset was delayed. Our results may provide insight into methods for better predicting and modulating IR of SAA patients and subsequently improving outcomes after transplantation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brodsky RA, Jones RJ . Aplastic anaemia. Lancet 2005; 365: 1647–1656.
Young NS . Aplastic anaemia. Lancet 1995; 346: 228–232.
Marsh JCW . Management of acquired aplastic anaemia. Blood Rev 2005; 19: 143–151.
Armand P, Antin JH . Allogeneic stem cell transplantation for aplastic anemia. Biol Blood Marrow Transplant 2007; 13: 505–516.
Arai Y, Kondo T, Yamazaki H, Takenaka K, Sugita J, Kobayashi T et al. Allogeneic unrelated bone marrow transplantation from older donors results in worse prognosis in recipients with aplastic anemia. Haematologica 2016; 101: 644–652.
Bacigalupo A . Bone marrow transplantation for acquired severe aplastic anemia. Hematol-Oncol Clin North Am 2014; 28: 1145-+.
Ciceri F, Lupo-Stanghellini MT, Korthof ET, Saa-Wp E . Haploidentical transplantation in patients with acquired aplastic anemia. Bone Marrow Transplant 2013; 48: 183–185.
Zhu H, Luo RM, Luan Z, Lee V, Zhu YP, Luo CJ et al. Unmanipulated haploidentical haematopoietic stem cell transplantation for children with severe aplastic anaemia. Br. J. Haematol 2016; 174: 799–805.
Xu LP, Liu KY, Liu DH, Han W, Chen H, Chen YH et al. A novel protocol for haploidentical hematopoietic SCT without in vitro T-cell depletion in the treatment of severe acquired aplastic anemia. Bone Marrow Transplant 2012; 47: 1507–1512.
Xu LP, Wang SQ, Wu DP, Wang JM, Gao SJ, Jiang M et al. Haplo-identical transplantation for acquired severe aplastic anaemia in a multicentre prospective study. Br J Haematol 2016; 175: 265–274.
Seggewiss R, Einsele H . Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood 2010; 115: 3861–3868.
Lucarelli B, Merli P, Bertaina V, Locatelli F . Strategies to accelerate immune recovery after allogeneic hematopoietic stem cell transplantation. Expert Rev Clin Immunol 2016; 12: 343–358.
Terasako K, Sato K, Sato M, Kimura S, Nakasone H, Okuda S et al. The effect of different ATG preparations on immune recovery after allogeneic hematopoietic stem cell transplantation for severe aplastic anemia. Hematology 2010; 15: 165–169.
Woodard P, Cunningham JM, Benaim E, Chen X, Hale G, Horwitz E et al. Effective donor lymphohematopoietic reconstitution after haploidentical CD34(+)-selected hematopoietic stem cell transplantation in children with refractory severe aplastic anemia. Bone Marrow Transplant 2004; 33: 411–418.
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W et al. Treatment of acute leukemia with unmanipulated HLA-mismatched/haploidentical blood and bone marrow transplantation. Biol Blood Marrow Transplant 2009; 15: 257–265.
Chang YJ, Zhao XY, Huo MR, Xu LP, Liu DH, Liu KY et al. Immune reconstitution following unmanipulated HLA-mismatched/haploidentical transplantation compared with HLA-identical sibling transplantation. J Clin Immunol 2012; 32: 268–280.
DeZern AE, Brodsky RA . Clinical management of aplastic anemia. Expert Rev Hematol 2011; 4: 221–230.
Marsh JC, Ball SE, Darbyshire P, Gordon-Smith EC, Keidan AJ, Martin A et al. Guidelines for the diagnosis and management of acquired aplastic anaemia. Br J Haematol 2003; 123: 782–801.
Chang YJ, Xu LP, Liu DH, Liu KY, Han W, Chen YH et al. The impact of CD34+ cell dose on platelet engraftment in pediatric patients following unmanipulated haploidentical blood and marrow transplantation. Pediatr Blood Cancer 2009; 53: 1100–1106.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–956.
Xhaard A, Moins-Teisserenc H, Busson M, Robin M, Ribaud P, Dhedin N et al. Reconstitution of regulatory T-cell subsets after allogeneic hematopoietic SCT. Bone Marrow Transplant 2014; 49: 1089–1092.
Kim HO, Oh HJ, Lee JW, Jang PS, Chung NG, Cho B et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation in children: a single institution study of 59 patients. Korean J Pediatr 2013; 56: 26–31.
Petersen SL, Ryder LP, Bjork P, Madsen HO, Heilmann C, Jacobsen N et al. A comparison of T-, B- and NK-cell reconstitution following conventional or nonmyeloablative conditioning and transplantation with bone marrow or peripheral blood stem cells from human leucocyte antigen identical sibling donors. Bone Marrow Transplant 2003; 32: 65–72.
Bosch M, Dhadda M, Hoegh-Petersen M, Liu Y, Hagel LM, Podgorny P et al. Immune reconstitution after anti-thymocyte globulin-conditioned hematopoietic cell transplantation. Cytotherapy 2012; 14: 1258–1275.
Koh K-N, Im HJ, Kim BE, Choi ES, Jang S, Kwon SW et al. Haploidentical haematopoietic stem cell transplantation using CD3 or CD3/CD19 depletion and conditioning with fludarabine, cyclophosphamide and antithymocyte globulin for acquired severe aplastic anaemia. Br J Haematol 2012; 157: 139–142.
Im HJ, Koh KN, Choi ES, Jang S, Kwon SW, Park C-J et al. Excellent outcome of haploidentical hematopoietic stem cell transplantation in children and adolescents with acquired severe aplastic anemia. Biol Blood Marrow Transplant. 2013; 19: 754–759.
Gao L, Li Y, Zhang Y, Chen X, Gao L, Zhang C et al. Long-term outcome of HLA-haploidentical hematopoietic SCT without in vitro T-cell depletion for adult severe aplastic anemia after modified conditioning and supportive therapy. Bone Marrow Transplant 2014; 49: 519–524.
Forbes GM, Erber WN, Herrmann RP, Davies JM, Collins BJ . Immunohistochemical changes in sigmoid colon after allogeneic and autologous bone marrow transplantation. J Clin Pathol 1995; 48: 308–313.
Huttunen P, Taskinen M, Siitonen S, Saarinen-Pihkala UM . Impact of very early CD4(+)/CD8(+) T cell counts on the occurrence of acute graft-versus-host disease and NK cell counts on outcome after pediatric allogeneic hematopoietic stem cell transplantation. Pediatr Blood Cancer 2015; 62: 522–528.
Martins SL St, John LS, Champlin RE, Wieder ED, McMannis J, Molldrem JJ et al. Functional assessment and specific depletion of alloreactive human T cells using flow cytometry. Blood 2004; 104: 3429–3436.
Luo XH, Chang YJ, Xu LP, Liu DH, Liu KY, Huang XJ . The impact of graft composition on clinical outcomes in unmanipulated HLA-mismatched/haploidentical hematopoietic SCT. Bone Marrow Transplant 2009; 43: 29–36.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81370666 and No. 81530046). The authors thank every faculty member of Peking University People’s Hospital, Institute of Hematology who has participated in this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pei, XY., Zhao, XY., Xu, LP. et al. Immune reconstitution in patients with acquired severe aplastic anemia after haploidentical stem cell transplantation. Bone Marrow Transplant 52, 1556–1562 (2017). https://doi.org/10.1038/bmt.2017.174
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2017.174
This article is cited by
-
Thiotepa-based reduced toxicity conditioning in combination with post-transplant cyclophosphamide and mTOR inhibitor for heavily transfused acquired severe aplastic anemia in children and young adults: encouraging outcomes of a pilot study
Bone Marrow Transplantation (2023)
-
Mesenchymal stromal cells as prophylaxis for graft-versus-host disease in haplo-identical hematopoietic stem cell transplantation recipients with severe aplastic anemia?—a systematic review and meta-analysis
Stem Cell Research & Therapy (2021)
-
Haploidentical stem cell transplantation for aplastic anemia: the current advances and future challenges
Bone Marrow Transplantation (2021)