Abstract
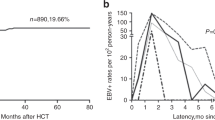
EBV infection is one of the life-threatening clinical complications in patients who underwent haploidentical hematopoietic stem cell transplantation (haploHSCT). Although immune recovery is recognized to be crucial for decreasing subsequent morbidity of infections, the link between T-cell recovery and EBV infection after haploHSCT remains elusive. We recently compared the influences of different doses of antithymocyte globulin conditioning on the T-cell reconstitution post haploHSCT and suggested that CD4−CD8−T cells might interact with the occurrence of EBV reactivation. In the current study, haploHSCT recipients with EBV-DNAemia (n=64) were compared with a control group without EBV reactivation (n=192), with regard to the recoveries of T-cell subpopulations. In contrast to other T-cell subpopulations, the median counts ofCD4−CD8−T cells in recipients with EBV-DNAemia were significantly lower than the control group at a serial time course (30, 90 and 180 days) after transplantation. Landmark studies further confirmed the correlation of CD4−CD8−T cells with the EBV infection. Multivariate analysis showed that hampered recovery of CD4−CD8−T cells and EBV reactivation were the independent risk factors to predict transplant-related mortality. Our findings may facilitate the intervention strategies to improve the overall survival of haploHSCT recipients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Huang XJ, Zhu HH, Chang YJ, Xu LP, Liu DH, Zhang XH et al. The superiority of haploidentical related stem cell transplantation over chemotherapy alone as postremission treatment for patients with intermediate- or high-risk acute myeloid leukemia in first complete remission. Blood 2012; 119: 5584–5590.
Wang Y, Chang YJ, Xu LP, Liu KY, Liu DH, Zhang XH et al. Who is the best donor for a related HLA haplotype-mismatched transplant? Blood 2014; 124: 843–850.
Kasamon YL, Bolanos-Meade J, Prince GT, Tsai HL, McCurdy SR, Kanakry JA et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol 2015; 33: 3152–3161.
Ocheni S, Kroeger N, Zabelina T, Sobottka I, Ayuk F, Wolschke C et al. EBV reactivation and post transplant lymphoproliferative disorders following allogeneic SCT. Bone Marrow Transplant 2008; 42: 181–186.
Peric Z, Cahu X, Chevallier P, Brissot E, Malard F, Guillaume T et al. Features of Epstein-Barr Virus (EBV) reactivation after reduced intensity conditioning allogeneic hematopoietic stem cell transplantation. Leukemia 2011; 25: 932–938.
Han TT, Xu LP, Liu DH, Liu KY, Zhang XH, Chen H et al. [Prevalence of EBV infection in patients with allogeneic hematopoietic stem cell transplantation]. Zhonghua xue ye xue za zhi 2013; 34: 651–654.
Xu LP, Zhang CL, Mo XD, Zhang XH, Chen H, Han W et al. Epstein-Barr virus-related post-transplantation lymphoproliferative disorder after unmanipulated human leukocyte antigen haploidentical hematopoietic stem cell transplantation: incidence, risk factors, treatment, and clinical outcomes. Biol Blood Marrow Transplant 2015; 21: 2185–2191.
Seggewiss R, Einsele H . Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood 2010; 115: 3861–3868.
Forcina A, Noviello M, Carbone MR, Bonini C, Bondanza A . Predicting the clinical outcome of allogeneic hematopoietic stem cell transplantation: the long and winding road toward validated immune biomarkers. Front Immunol 2013; 4: 71.
Burns DM, Tierney R, Shannon-Lowe C, Croudace J, Inman C, Abbotts B et al. Memory B-cell reconstitution following allogeneic hematopoietic stem cell transplantation is an EBV-associated transformation event. Blood 2015; 126: 2665–2675.
Meij P, van Esser JW, Niesters HG, van Baarle D, Miedema F, Blake N et al. Impaired recovery of Epstein-Barr virus (EBV)—specific CD8+ T lymphocytes after partially T-depleted allogeneic stem cell transplantation may identify patients at very high risk for progressive EBV reactivation and lymphoproliferative disease. Blood 2003; 101: 4290–4297.
Comoli P, Zecca M, Maccario R . Immunotherapy against EBV-lymphoma in recipients of HSCT. Expert Rev Hematology 2010; 3: 625–632.
Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 2010; 115: 925–935.
Peggs KS . Reconstitution of adaptive and innate immunity following allogeneic hematopoietic stem cell transplantation in humans. Cytotherapy 2006; 8: 427–436.
Liu J, Xu LP, Bian Z, Chang YJ, Wang Y, Zhang XH et al. Differential impact of two doses of antithymocyte globulin conditioning on lymphocyte recovery upon haploidentical hematopoietic stem cell transplantation. J Transl Med 2015; 13: 391.
Wang Y, Fu HX, Liu DH, Xu LP, Zhang XH, Chang YJ et al. Influence of two different doses of antithymocyte globulin in patients with standard-risk disease following haploidentical transplantation: a randomized trial. Bone Marrow Transplant 2014; 49: 426–433.
Fallen PR, McGreavey L, Madrigal JA, Potter M, Ethell M, Prentice HG et al. Factors affecting reconstitution of the T cell compartment in allogeneic haematopoietic cell transplant recipients. Bone Marrow Transplant 2003; 32: 1001–1014.
Bosch M, Khan FM, Storek J . Immune reconstitution after hematopoietic cell transplantation. Curr Opin Hematol 2012; 19: 324–335.
Priatel JJ, Utting O, Teh HS . TCR/self-antigen interactions drive double-negative T cell peripheral expansion and differentiation into suppressor cells. J Immunol 2001; 167: 6188–6194.
Hillhouse EE, Lesage S . A comprehensive review of the phenotype and function of antigen-specific immunoregulatory double negative T cells. J Autoimmun 2013; 40: 58–65.
Crispin JC, Tsokos GC . Human TCR-alpha beta+ CD4−– CD8−– T cells can derive from CD8+ T cells and display an inflammatory effector phenotype. J Immunol 2009; 183: 4675–4681.
Juvet SC, Zhang L . Double negative regulatory T cells in transplantation and autoimmunity: recent progress and future directions. J Mol Cell Biol 2012; 4: 48–58.
D'Acquisto F, Crompton T . CD3+CD4-CD8- (double negative) T cells: saviours or villains of the immune response? Biochem Pharmacol 2011; 82: 333–340.
Milush JM, Mir KD, Sundaravaradan V, Gordon SN, Engram J, Cano CA et al. Lack of clinical AIDS in SIV-infected sooty mangabeys with significant CD4+ T cell loss is associated with double-negative T cells. J Clin Investig 2011; 121: 1102–1110.
Petitjean G, Chevalier MF, Tibaoui F, Didier C, Manea ME, Liovat AS et al. Level of double negative T cells, which produce TGF-beta and IL-10, predicts CD8 T-cell activation in primary HIV-1 infection. AIDS 2012; 26: 139–148.
Sundaravaradan V, Mir KD, Sodora DL . Double-negative T cells during HIV/SIV infections: potential pinch hitters in the T-cell lineup. Curr Opin HIV AIDS 2012; 7: 164–171.
Acknowledgements
This study is supported by Key Program of the National Natural Science Foundation of China (Grant No 81230013) and National Natural Science Foundation of China (Grant No. 81370666).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bian, Z., Liu, J., Xu, LP. et al. Association of Epstein–Barr virus reactivation with the recovery of CD4/CD8 double-negative T lymphocytes after haploidentical hematopoietic stem cell transplantation. Bone Marrow Transplant 52, 264–269 (2017). https://doi.org/10.1038/bmt.2016.238
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2016.238
This article is cited by
-
The time-dependent effects of early-onset Epstein-Barr viremia on adult acute leukemia patients following allo-HSCT with ATG-containing MAC regimen
Annals of Hematology (2021)
-
Update of the “Beijing Protocol” haplo-identical hematopoietic stem cell transplantation
Bone Marrow Transplantation (2019)
-
Incidence, risk factors, and clinical significance of Epstein–Barr virus reactivation in myelodysplastic syndrome after allogeneic haematopoietic stem cell transplantation
Annals of Hematology (2019)