Abstract
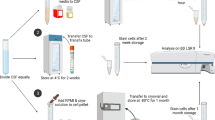
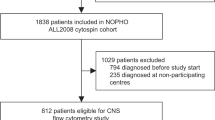
Central nervous system (CNS) complications have been described in patients undergoing allogeneic hematopoietic cell transplantation (alloHCT). Cerebrospinal fluid (CSF) analysis is included in the diagnostic workup in patients with neurological symptoms after alloHCT. CSF donor–recipient chimerism analysis usually is not used to evaluate patients with neurological complications after alloHCT. To assess the potential contribution of CSF donor–recipient chimerism in patients with neurological complications, we analyzed 85 CSF samples from 50 patients with neurological complications after alloHCT. After alloHCT, 21 patients showed the presence of recipient-derived DNA. In 13 of these patients, recurrence of the underlying disease was detected in CSF. There was a moderate correlation between the recipient DNA percentage as detected by short tandem repeat (STR) amplification and the cell concentration in CSF (Spearmann r: 0.66 P=0.004). The percentage of cells with immunophenotypic abnormalities from patients relapsing in the CNS detected by flow cytometry showed a strong correlation with the percentage of recipient-derived DNA in CSF assessed by STR analysis (Spearmann r: 0.83 P=0.0008). Donor–recipient chimerism analysis in CSF in patients with neurological symptoms after alloHCT is a practical, feasible and useful complementary method to the already established methodologies included in the diagnostic workup.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Siegal D, Keller A, Xu W, Bhuta S, Kim DH, Kuruvilla J et al. Central nervous system complications after allogeneic hematopoietic stem cell transplantation: incidence, manifestations and clinical significance. Biol Blood Marrow Transplant 2007; 13: 1369–1379.
Hamdi A, Mawad R, Bassett R, di Stasi A, Ferro R, Afrough A et al. Central nervous system relapse in adults with acute lymphoblastic leukemia after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2014; 20: 1767–1771.
Glantz MJ, Cole BF, Glantz LK, Cobb J, Mills P, Lekos A et al. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer 1998; 82: 733–739.
Bromberg JE, Breems DA, Kraan J, Bikker G, van der Holt B, Smitt PS et al. CSF flow cytometry greatly improves diagnostic accuracy in CNS hematologic malignancies. Neurology 2007; 68: 1674–1679.
Liu L, Cao F, Wang S, Zhou J, Yang G, Wang C . Detection of malignant B lymphocytes by PCR clonality assay using direct lysis of cerebrospinal fluid and low volume specimens. Int J Lab Hematol 2014; 37: 165–173.
Baehring JM, Hochberg FH, Betensky RA, Longtine J, Sklar J . Immunoglobulin gene rearrangement analysis in cerebrospinal fluid of patients with lymphoproliferative processes. J Neurol Sci 2006; 247: 208–216.
Hibi S, Tsunamoto K, Todo S, Sawada T, Ueda Y, Taniwaki M et al. Chimerism analysis on mononuclear cells in the CSF after allogeneic bone marrow transplantation. Bone Marrow Transplant 1997; 20: 503–506.
Ohashi H, Kato C, Fukami S, Saito H, Hamaguchi M . Leukemic relapse in the central nervous system after allogeneic stem cell transplantation with complete remission in the bone marrow and donor-type chimerism: report of two cases. Am J Hematol 2005; 79: 142–146.
Subira D, Castañon S, Roman A, Aceituno E, Jimenez-Garofano C, Jimenez A et al. Flow cytometry and the study of central nervous disease in patients with acute leucemia. Br J Haematol 2001; 112: 381–384.
Roma AA, Garcia A, Avagnina A, Rescia C, Elsner B . Lymphoid and myeloid neoplasm involving cerebrospinal fluid: comparison of morphologic examination and immunophenotyping by flow cytometry. Diagn Cytopathol 2002; 27: 271–275.
Marks R, Potthoff K, Hahn J, Ihorst G, Bertz H, Spyridonidis A et al. Reduced-toxicity conditioning with fludarabine, BCNU and melphalan in allogeneic cell transplantation: particular activity against advanced hematologic malignancies. Blood 2008; 112: 415–425.
Spyridonidis A, Zeiser R, Wäsch R, Bertz H, Finke J . Capillary electrophoresis for chimerism monitoring by PCR amplification of microsatellite markers after allogeneic hematopoietic cell transplantation. Clin Transplant 2005; 19: 350–356.
Kristt D, Klein T . Reliability of quantitative chimerism results: assessment of sample performance using novel parameters. Leukemia 2006; 20: 1169–1172.
Oelschlägel U, Nowak R, Schaub A, Köppel C, Herbst R, Mohr B et al. Shift of aberrant antigen expression at relapse or at treatment failure in acute leukemia. Cytometry 2000; 42: 247–253.
Crespo-Solis E, Lopez-Karpovitch X, Higuera J, Vega-Ramos B . Diagnosis of acute leukemia in cerebrospinal fluid. Curr Oncol Rep 2012; 14: 369–378.
de Graaf MT, de Jongste AH, Kraan J, Boostra JG, Sillevis Smitt PA, Gratama JW . Flow cytometric characterization of cerebrospinal fluid cells. Cytometry B Clin Cytom 2011; 80: 271–281.
Dissing M, Le Beau MM, Pedersen-Bjergaard J . Inversion of chromosome 16 and uncommon rearrangements of the CBFB and MYH11 genes in therapy-related acute myeloid leukemia: rare events related to DNA-topoisomerase II inhibitors? J Clin Oncol 1998; 16: 1890–1896.
Waterhouse M, Kunzmann R, Torres M, Bertz H, Finke J . An internal validation approach and quality control on hematopoieitc chimerism testing after allogeneic hematopoietic cell transplantation. Clin Chem Lab Med 2013; 51: 363–369.
van den Berg H, Gerritsen EJ, Haraldsson A, Vossen JM . Changes in cell and protein content of cerebrospinal fluid in children with acute lymphoblastic leukaemia after allogeneic bone marrow transplantation. Bone Marrow Transplant 1993; 12: 615–619.
Acknowledgements
We are grateful to the transplantation team at Freiburg University Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Rights and permissions
About this article
Cite this article
Waterhouse, M., Bartsch, I., Bertz, H. et al. Cerebrospinal fluid chimerism analysis in patients with neurological symptoms after allogeneic cell transplantation. Bone Marrow Transplant 51, 127–131 (2016). https://doi.org/10.1038/bmt.2015.226
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2015.226