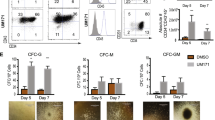
Abstract
The influence of TNF-α and Fas-ligand (FasL) on viability and function was evaluated in fresh- and expanded-umbilical cord blood (UCB) cells. CD34+ progenitors and T cells display outstanding survival, whereas ~30% and >50% B lymphocytes and myeloid cells undergo spontaneous apoptosis within 24 and 48 h, respectively. Although the impact of exposure to toxic doses of FasL and TNF-α was undetectable in measurements of apoptosis; removal of dead cells after 2 days of incubation with the ligands revealed a twofold increase in frequency of colony-forming cells (CFU). The sensitivity of progenitors to apoptosis was also unaffected by Fas cross-linking following TNF-induced upregulation of the receptor, increasing CFU frequency without impairing SCID repopulating cell (SRC) activity. Most significant enrichment in CD34+ progenitors and corresponding increase in CFU frequency were observed when FasL was applied during the final week of ex vivo expansion under the influence of nicotinamide, without impairing SRC activity. These data emphasize differential sensitivities of UCB progenitors and lineage-positive cells to apoptotic signaling mediated by the Fas and TNF receptors, which might be useful in improving the efficiency of ex vivo expansion and improving UCB cell engraftment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fernández MN, Millán I, Gluckman E . Cord-blood transplants. New Engl J Med 1999; 340: 1287–1288.
Rosler ES, Brandt JE, Chute J, Hoffman R . An in vivo competitive repopulation assay for various sources of human hematopoietic stem cells. Blood 2000; 96: 3414–3421.
Tanavde VM, Malehorn MT, Lumkul R, Gao Z, Wingard J, Garrett ES et al. Human stem-progenitor cells from neonatal cord blood have greater hematopoietic expansion capacity than those from mobilized adult blood. Exp Hematol 2002; 30: 816–823.
Frassoni F, Podesta M, Maccario R, Giorgiani G, Rossi G, Zecca M et al. Cord blood transplantation provides better reconstitution of hematopoietic reservoir compared with bone marrow transplantation. Blood 2003; 102: 1138–1141.
Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. New Engl J Med 2004; 351: 2276–2285.
Lekakis L, Giralt S, Couriel D, Shpall EJ, Hosing C, Khouri IF et al. Phase II study of unrelated cord blood transplantation for adults with high-risk hematologic malignancies. Bone Marrow Transplant 2006; 38: 421–426.
Tse W, Laughlin M . Cord blood transplantation in adult patients. Cytotherapy 2005; 7: 228–242.
Schoemans H, Theunissen K, Maertens J, Boogaerts M, Verfaillie C, Wagner J . Adult umbilical cord blood transplantation: a comprehensive review. Bone Marrow Transplant 2006; 38: 83–93.
Brown JA, Boussiotis VA . Umbilical cord blood transplantation: basic biology and clinical challenges to immune reconstitution. Clin Immunol 2008; 127: 286–297.
Rocha V, Broxmeyer H . New approaches for improving engraftment after Cord Blood Transplantation. Biol Blood Marrow Transplant 2010; 16 (Suppl 1): S126–S132.
Barker JN, Weisdorf DJ, DeFor TE, Barker JN, Weisdorf DJ, DeFor TE et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood 2005; 105: 1343–1347.
Brunstein CG, Barker JN, Weisdorf DJ, Defor TE, McKenna D, Chong SY et al. Intra-BM injection to enhance engraftment after myeloablative umbilical cord blood transplantation with two partially HLA-matched units. Bone Marrow Transplant 2009; 43: 935–940.
Peled T, Landau E, Mandel J, Glukhman E, Goudsmid NR, Nagler A et al. Linear polyamine copper chelator tetraethylenepentamine augments long-term ex vivo expansion of cord blood-derived CD34+ cells and increases their engraftment potential in NOD/SCID mice. Exp Hematol 2004; 32: 547–555.
Araki H, Mahmud N, Milhem M, Nunez R, Xu M, Beam CA et al. Expansion of human umbilical cord blood SCID-repopulating cells using chromatin-modifying agents. Exp Hematol 2006; 34: 140–149.
de Lima M, McMannis J, Gee A, Komanduri K, Couriel D, Andersson BS et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: a phase I/II clinical trial. Bone Marrow Transplant 2008; 41: 771–778.
Rocha V, Wagner JE Jr, Sobocinski KA, Klein JP, Zhang MJ, Horowitz MM et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. New Engl J Med 2000; 342: 1846–1854.
Pearl-Yafe M, Yolcu ES, Stein J, Kaplan O, Shirwan H, Yaniv I, Askenasy N . Expression of Fas and Fas-ligand in donor hematopoietic stem and progenitor cells is dissociated from the sensitivity to apoptosis. Exp Hematol 2007; 35: 1601–1612.
Pearl-Yafe M, Mizrahi K, Stein J, Stein J, Yolcu ES, Kaplan O et al. Tumor necrosis factor receptors support murine hematopoietic progenitor function in the early stages of engraftment. Stem Cells 2010; 28: 1270–1280.
Mizrahi K, Stein J, Pearl-Yafe M, Kaplan O, Yaniv I, Askenasy N . Regulatory functions of TRAIL in hematopoietic progenitors: human umbilical cord blood and murine bone marrow transplantation. Leukemia 2010; 24: 1325–1334.
Mizrahi K, Stein J, Kaplan O, Yaniv I, Zipori D, Askenasy N . Resistance of hematopoietic progenitors to Fas-mediated apoptosis is actively sustained by NFκB with a characteristic transcriptional signature. Stem Cell Dev 2013; 23: 676–686.
Pearl-Yafe M, Stein J, Yolcu ES, Farkas DL, Shirwan H, Yaniv I et al. Fas transduces dual apoptotic and trophic signals in hematopoietic progenitors. Stem Cells 2007; 25: 3194–3203.
Mizrahi K, Stein J, Yaniv I, Kaplan O, Askenasy N . TNF-α has tropic rather than apoptotic activity in human hematopoietic progenitors: involvement of TNF receptor-1 and caspase-8. Stem Cells 2013; 31: 156–166.
Saheki K, Fujimori Y, Takemoto Y, Kakishita E . Increased expression of Fas (APO-1, CD95) on CD34+ haematopoietic progenitor cells after allogeneic bone marrow transplantation. Br J Haematol 2000; 109: 447–452.
Pearl-Yafe M, Yolcu ES, Stein J, Kaplan O, Yaniv I, Shirwan H et al. Fas ligand enhances hematopoietic cell engraftment through abrogation of alloimmune responses and nonimmunogenic interactions. Stem Cells 2007; 25: 1448–1455.
Askenasy EM, Shushlav Y, Sun Z, Shirwan H, Yolcu ES, Askenasy N . Engineering of bone marrow cells with fas-ligand protein-enhances donor-specific tolerance to solid organs. Transplant Proc 2011; 43: 3545–3548.
Askenasy N, Ash S, Yaniv I, Stein J . Depletion of naïve lymphocytes with Fas ligand ex vivo prevents graft-versus-host disease without impairing T cell support of engraftment or graft-versus-tumor activity. Biol Blood Marrow Transplant 2013; 19: 185–195.
Mizrahi K, Yaniv I, Ash S, Stein J, Askenasy N . Apoptotic signaling through Fas and TNF receptors ameliorates GvHD in mobilized peripheral blood grafts. Bone Marrow Transplant (e-pub ahead of print 24 February 2014; doi:10.1038/bmt.2014.12).
Peled T, Shoham H, Aschengrau D, Yackoubov D, Frei G, Rosenheimer GN et al. Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol 2012; 40: 342–355.
Maciejewski J, Selleri C, Anderson S, Young NS . Fas antigen expression on CD34+ human marrow cells is induced by interferon gamma and tumor necrosis factor alpha and potentiates cytokine-mediated hematopoietic suppression in vitro. Blood 1995; 85: 3183–3190.
Bryder D, Ramsfjell V, Dybedal I, Theilgaard-Mönch K, Högerkorp CM, Adolfsson J et al. Self-renewal of multipotent long-term repopulating hematopoietic stem cells is negatively regulated by Fas and tumor necrosis factor receptor activation. J Exp Med 2001; 194: 941–952.
Guenechea G, Segovia JC, Albella B, Lamana M, Ramírez M, Regidor C et al. Delayed engraftment of nonobese diabetic severe combined immunodeficient mice transplanted with ex vivo–expanded human CD34 cord blood cells. Blood 1999; 93: 1097–1105.
Glimm H, Oh IH, Eaves CJ . Human hematopoietic stem cells stimulated to proliferate in vitro lose engraftment potential during their S/G(2)/ M transit and do not reenter G(0). Blood 2000; 96: 4185–4193.
Ratajczak J, Kucia M, Reca R, Zhang J, Machalinski B, Ratajczak MZ . Quiescent CD34+ early erythroid progenitors are resistant to several erythropoietic 'inhibitory' cytokines; role of FLIP. Br J Haematol 2003; 123: 160–169.
Takenaka K, Nagafuji K, Harada M, Mizuno S, Miyamoto T, Makino S et al. In vitro expansion of hematopoietic progenitor cells induces functional expression of Fas antigen (CD95). Blood 1996; 88: 2871–2877.
Santiago-Schwarz F, Borrero M, Tucci J, Palaia T, Carsons SE . In vitro expansion of CD13+CD33+ dendritic cell precursors from multipotent progenitors is regulated by a discrete fas-mediated apoptotic schedule. J Leukoc Biol 1997; 62: 493–502.
Askenasy N, Yaniv I, Stein J, Sharkis SJ . Our perception of developmental plasticity: esse est percipi (to be is to be perceived)? Curr Stem Cell Res Ther 2006; 1: 85–94.
Mizrahi K, Askenasy N . Activation and crosstalk between TNF family receptors in umbilical cord blood cells is not responsible for loss of engraftment capacity following culture. Am J Stem Cells 2013; 2: 155–164.
Frassoni F, Gualandi F, Podestà M, Raiola AM, Ibatici A, Piaggio G et al. Direct intrabone transplant of unrelated cord-blood cells in acute leukaemia: a phase I/II study. Lancet Oncol 2008; 9: 831–839.
Rocha V, Labopin M, Ruggeri A, Podestà M, Gallamini A, Bonifazi F et al. Unrelated cord blood transplantation: outcomes after single-unit intrabone injection compared with double-unit intravenous injection in patients with hematological malignancies. Transplantation 2013; 95: 1284–1291.
Harris DT, LoCascio J, Besencon FJ . Analysis of the alloreactive capacity of human umbilical cord blood: implications for graft-versus-host disease. Bone Marrow Transplant 1994; 14: 545–553.
Aggarwal S, Gupta A, Nagata S, Gupta S . Programmed cell death (apoptosis) in cord blood lymphocytes. J Clin Immunol 1997; 17: 63–73.
Sato K, Nagayama H, Takahashi TA . Aberrant CD3- and CD28-mediated signaling events in cord blood T cells are associated with dysfunctional regulation of Fas ligand-mediated cytotoxicity. J Immunol 1999; 162: 4464–4471.
Risdon G, Gaddy J, Stehman FB, Broxmeyer HE . Proliferative and cytotoxic responses of human cord blood T lymphocytes following allogeneic stimulation. Cell Immunol 1994; 154: 14–24.
El Ghalbzouri A, Drennou B, Blancheteau V . An in vitro model of allogeneic stimulation of cord blood: induction of Fas independent apoptosis. Hum Immunol 1999; 60: 598–607.
Lin SJ, Cheng PJ, Hsiao SS, Lin HH, Kuo ML . Differential effect of IL-15 and IL-2 on survival of PHA-activated umbilical cord blood T cells. Am J Hematol 2005; 80: 106–112.
Lin SJ, Lee CC, Cheng PJ, See LC, Kuo ML . Susceptibility to Fas and TNF-α receptor mediated apoptosis of anti-CD3/anti-CD28-activated umbilical cord blood T cells. Pediatr Allergy Immunol 2000; 20: 392–398.
Rocha V, Cornish J, Sievers EL, Filipovich A, Locatelli F, Peters C et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood 2001; 97: 2962–2971.
Rocha V, Broxmeyer HE . New approaches for improving engraftment after cord blood transplantation. Biol Blood Marrow Transplant 2010; 16 (Suppl. 1): S126–S132.
Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini I et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. New Engl J Med 1997; 337: 373–381.
Laughlin MJ, Barker J, Bambach B, Koc ON, Rizzieri DA, Wagner JE et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. New Engl J Med 2001; 344: 1815–1822.
Moscardó F, Sanz J, Senent L, Cantero S, de la Rubia J, Montesinos P et al. Impact of hematopoietic chimerism at day +14 on engraftment after unrelated donor umbilical cord blood transplantation for hematologic malignancies. Haematologica 2009; 94: 827–832.
Hamza NS, Lisgaris M, Yadavalli G, Nadeau L, Fox R, Fu P et al. Kinetics of myeloid and lymphocyte recovery and infectious complications after unrelated umbilical cord blood versus HLA-matched unrelated donor allogeneic transplantation in adults. Br J Haematol 2004; 124: 488–498.
Acknowledgements
This work was funded by grants from the Frankel Trust for Experimental Bone Marrow Transplantation. We thank Mrs Ela Zuzovsky and Mrs Ana Zemliansky for their outstanding technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Tony Peled has ownership interest in Gamida Cell. The other authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mizrahi, K., Ash, S., Peled, T. et al. Negative selection by apoptosis enriches progenitors in naïve and expanded human umbilical cord blood grafts. Bone Marrow Transplant 49, 942–949 (2014). https://doi.org/10.1038/bmt.2014.79
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2014.79