Abstract
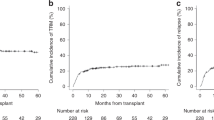
To elucidate the impact of pretransplant body mass index (BMI) on the clinical outcome, we performed a retrospective study with registry data including a total of 12 050 patients (age ⩾18 years) who received allogeneic hematopoietic SCT (HSCT) between 2000 and 2010. Patients were stratified as follows: BMI<18.5 kg/m2, Underweight, n=1791; 18.5⩽BMI<25, Normal, n=8444; 25⩽BMI<30, Overweight, n=1591; BMI⩾30, Obese, n=224. The median age was 45 years (range, 18–77). A multivariate analysis showed that the risk of relapse was significantly higher in the underweight group and lower in the overweight and obese groups compared with the normal group (hazard ratio (HR), 1.16, 0.86, and 0.74, respectively). The risk of GVHD was significantly higher in the overweight group compared with the normal group. The risk of non-relapse mortality (NRM) was significantly higher in the overweight and obese group compared with the normal group (HR 1.19 and HR 1.43, respectively). The probability of OS was lower in the underweight group compared with the normal group (HR 1.10, P=0.018). In conclusion, pretransplant BMI affected the risk of relapse and NRM after allogeneic HSCT. Underweight was a risk factor for poor OS because of an increased risk of relapse. Obesity was a risk factor for NRM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011; 377: 557–567.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011; 12: 489–495.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106: 2912–2919.
Navarro WH, Agovi MA, Logan BR, Ballen K, Bolwell BJ, Frangoul H et al. Obesity does not preclude safe and effective myeloablative hematopoietic cell transplantation (HCT) for acute myelogenous leukemia (AML) in adults. Biol Blood Marrow Transplant 2010; 16: 1442–1450.
Fuji S, Kim SW, Yoshimura K, Akiyama H, Okamoto S, Sao H et al. Possible association between obesity and posttransplantation complications including infectious diseases and acute graft-versus-host disease. Biol Blood Marrow Transplant 2009; 15: 73–82.
Atsuta Y, Suzuki R, Yoshimi A, Gondo H, Tanaka J, Hiraoka A et al. Unification of hematopoietic stem cell transplantation registries in Japan and establishment of the TRUMP System. Int J Hematol 2007; 86: 269–274.
WHO. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000; 894: 1–253.
Pi-Sunyer FX BD, Bouchard C, Carleton RA, Colditz GA, Dietz WH, Foreyt JP et al. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults–The Evidence Report. National Institutes of Health. Obes Res 1998; 6 (Suppl 2): 51S–209S.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18: 295–304.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5: 649–655.
Kanda Y . Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013; 48: 452–458.
Lee KH, Choi SJ, Lee JH, Lee JS, Kim WK, Lee KB et al. Prognostic factors identifiable at the time of onset of acute graft-versus-host disease after allogeneic hematopoietic cell transplantation. Haematologica 2005; 90: 939–948.
Robin M, Porcher R, de Castro R, Fisher G, de Latour RP, Ribaud P et al. Initial liver involvement in acute GVHD is predictive for nonrelapse mortality. Transplantation 2009; 88: 1131–1136.
Murata M, Nakasone H, Kanda J, Nakane T, Furukawa T, Fukuda T et al. Clinical factors predicting the response of acute graft-versus-host disease to corticosteroid therapy: an analysis from the GVHD Working Group of the Japan Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant 2013; 19: 1183–1189.
Weisdorf D, Haake R, Blazar B, Miller W, McGlave P, Ramsay N et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood 1990; 75: 1024–1030.
Ferrara JL, Levine JE, Reddy P, Holler E . Graft-versus-host disease. Lancet 2009; 373: 1550–1561.
Schipper HS, Prakken B, Kalkhoven E, Boes M . Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol Metab 2012; 23: 407–415.
Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 2005; 11: 183–190.
Conde J, Scotece M, Gomez R, Lopez V, Gomez-Reino JJ, Lago F et al. Adipokines: biofactors from white adipose tissue. A complex hub among inflammation, metabolism, and immunity. Biofactors 2011; 37: 413–420.
Heinbokel T, Floerchinger B, Schmiderer A, Edtinger K, Liu G, Elkhal A et al. Obesity and its impact on transplantation and alloimmunity. Transplantation 2013; 96: 10–16.
Oh H, Loberiza FR Jr, Zhang MJ, Ringden O, Akiyama H, Asai T et al. Comparison of graft-versus-host-disease and survival after HLA-identical sibling bone marrow transplantation in ethnic populations. Blood 2005; 105: 1408–1416.
Hahn T, McCarthy PL Jr, Zhang MJ, Wang D, Arora M, Frangoul H et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol 2008; 26: 5728–5734.
Baffy G . Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol 2009; 51: 212–223.
Tilg H, Moschen A . Weight loss: cornerstone in the treatment of non-alcoholic fatty liver disease. Minerva Gastroenterol Dietol 2010; 56: 159–167.
Musso G, Cassader M, Rosina F, Gambino R . Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia 2012; 55: 885–904.
Le Blanc K, Ringden O, Remberger M . A low body mass index is correlated with poor survival after allogeneic stem cell transplantation. Haematologica 2003; 88: 1044–1052.
Deeg HJ, Seidel K, Bruemmer B, Pepe MS, Appelbaum FR . Impact of patient weight on non-relapse mortality after marrow transplantation. Bone Marrow Transplant 1995; 15: 461–468.
Murry DJ, Riva L, Poplack DG . Impact of nutrition on pharmacokinetics of anti-neoplastic agents. Int J Cancer Suppl 1998; 11: 48–51.
Boullata JI . Drug disposition in obesity and protein-energy malnutrition. Proc Nutr Soc 2010; 69: 543–550.
Powis G, Reece P, Ahmann DL, Ingle JN . Effect of body weight on the pharmacokinetics of cyclophosphamide in breast cancer patients. Cancer Chemother Pharmacol 1987; 20: 219–222.
Kondrup J, Allison SP, Elia M, Vellas B, Plauth M . Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN) ESPEN guidelines for nutrition screening 2002. Clin Nutr 2003; 22: 415–421.
Coss CC, Bohl CE, Dalton JT . Cancer cachexia therapy: a key weapon in the fight against cancer. Curr Opin Clin Nutr Metab Care 2011; 14: 268–273.
Bosaeus I . Nutritional support in multimodal therapy for cancer cachexia. Support Care Cancer 2008; 16: 447–451.
Morishita S, Kaida K, Tanaka T, Itani Y, Ikegame K, Okada M et al. Prevalence of sarcopenia and relevance of body composition, physiological function, fatigue, and health-related quality of life in patients before allogeneic hematopoietic stem cell transplantation. Support Care Cancer 2012; 20: 3161–3168.
Kondrup J, Rasmussen HH, Hamberg O, Stanga Z . Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 2003; 22: 321–336.
Stratton RJ, Hackston A, Longmore D, Dixon R, Price S, Stroud M et al. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the 'malnutrition universal screening tool' ('MUST') for adults. Br J Nutr 2004; 92: 799–808.
Hoffmeister PA, Storer BE, Macris PC, Carpenter PA, Baker KS . Relationship of body mass index and arm anthropometry to outcomes after pediatric allogeneic hematopoietic cell transplantation for hematologic malignancies. Biol Blood Marrow Transplant 2013; 19: 1081–1086.
Acknowledgements
We thank the medical, nursing, data-processing, laboratory and clinical staffs at the participating centers for their important contributions to this study and their dedicated care of the patients. This study was supported in part by grants from the Ministry of Health, Labor and Welfare, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
Fuji, S., Takano, K., Mori, T. et al. Impact of pretransplant body mass index on the clinical outcome after allogeneic hematopoietic SCT. Bone Marrow Transplant 49, 1505–1512 (2014). https://doi.org/10.1038/bmt.2014.178
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2014.178
This article is cited by
-
Analysis of risk factors for fatal renal complications after allogeneic hematopoietic cell transplantation
Bone Marrow Transplantation (2024)
-
Lessons learned from early closure of a clinical trial for steroid-refractory acute GVHD
Bone Marrow Transplantation (2022)
-
Correlation of nutrition-associated parameters with non-relapse mortality in allogeneic hematopoietic stem cell transplantation
Annals of Hematology (2022)
-
Superior survival with pediatric-style chemotherapy compared to myeloablative allogeneic hematopoietic cell transplantation in older adolescents and young adults with Ph-negative acute lymphoblastic leukemia in first complete remission: analysis from CALGB 10403 and the CIBMTR
Leukemia (2021)
-
Feasibility and safety of exercise training and nutritional support prior to haematopoietic stem cell transplantation in patients with haematologic malignancies
BMC Cancer (2020)