Abstract
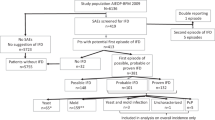
The objective of this study was to evaluate the efficacy and safety of micafungin for the prevention of invasive fungal infection (IFI) during the neutropenic phase of allogeneic hematopoietic SCT (allo-HSCT) in children and adolescents. This was a prospective, multicenter, open-label, single-arm study. Micafungin was administered i.v. at a dose of 1 mg/kg/day (max 50 mg) from the beginning of conditioning until neutrophil engraftment. Treatment success was defined as the absence of proven, probable, possible or suspected IFI through to 4 weeks after therapy. From April 2010 to December 2011, 155 patients were enrolled from 11 institutions in Korea, and 147 patients were analyzed. Of the 147 patients, 121 (82.3%) completed the protocol without premature interruption. Of the 132 patients in whom micafungin efficacy could be evaluated, treatment success was achieved in 119 patients (90.2%). There was no proven fungal infection in any patient. The number of patients with probable, possible and suspected IFI was two, two and nine, respectively. Thirty-five patients (23.8%) experienced 109 adverse events (AEs) possibly related to micafungin. No patients experienced grade IV AEs. Two patients (1.4%) discontinued micafungin administration due to adverse effects. None of the deaths were related to the study drug.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Marr KA, Carter RA, Crippa F, Wald A, Corey L . Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis 2002; 34: 909–917.
Kume H, Yamazaki T, Abe M, Tanuma H, Okudaira M, Okayasu I . [Epidemiology of visceral mycoses in patients with leukemia and MDS—Analysis of the data in annual of pathological autopsy cases in Japan in 1989, 1993, 1997 and 2001]. Nihon Ishinkin Gakkai Zasshi 2006; 47: 15–24.
Denning DW . Echinocandin antifungal drugs. Lancet 2003; 362: 1142–1151.
Yanada M, Kiyoi H, Murata M, Suzuki M, Iwai M, Yokozawa T et al. Micafungin, a novel antifungal agent, as empirical therapy in acute leukemia patients with febrile neutropenia. Intern Med 2006; 45: 259–264.
Kuse ER, Chetchotisakd P, da Cunha CA, Ruhnke M, Barrios C, Raghunadharao D et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 2007; 369: 1519–1527.
van Burik JA, Ratanatharathorn V, Stepan DE, Miller CB, Lipton JH, Vesole DH et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis 2004; 39: 1407–1416.
Hashino S, Morita L, Takahata M, Onozawa M, Nakagawa M, Kawamura T et al. Administration of micafungin as prophylactic antifungal therapy in patients undergoing allogeneic stem cell transplantation. Int J Hematol 2008; 87: 91–97.
Kusuki S, Hashii Y, Yoshida H, Takizawa S, Sato E, Tokimasa S et al. Antifungal prophylaxis with micafungin in patients treated for childhood cancer. Pediatr Blood Cancer 2009; 53: 605–609.
De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46: 1813–1821.
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239–245.
Maertens J, Theunissen K, Verhoef G, Verschakelen J, Lagrou K, Verbeken E et al. Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: a prospective feasibility study. Clin Infect Dis 2005; 41: 1242–1250.
Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 2006; 43: 25–31.
Hachem R, Hanna H, Kontoyiannis D, Jiang Y, Raad I . The changing epidemiology of invasive candidiasis: Candida glabrata and Candida krusei as the leading causes of candidemia in hematologic malignancy. Cancer 2008; 112: 2493–2499.
Kolve H, Ahlke E, Fegeler W, Ritter J, Jurgens H, Groll AH . Safety, tolerance and outcome of treatment with liposomal amphotericin B in paediatric patients with cancer or undergoing haematopoietic stem cell transplantation. J Antimicrob Chemother 2009; 64: 383–387.
Roden MM, Nelson LD, Knudsen TA, Jarosinski PF, Starling JM, Shiflett SE et al. Triad of acute infusion-related reactions associated with liposomal amphotericin B: analysis of clinical and epidemiological characteristics. Clin Infect Dis 2003; 36: 1213–1220.
Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N Engl J Med 1999; 340: 764–771.
Huang X, Chen H, Han M, Zou P, Wu D, Lai Y et al. Multicenter, randomized, open-label study comparing the efficacy and safety of micafungin versus itraconazole for prophylaxis of invasive fungal infections in patients undergoing hematopoietic stem cell transplant. Biol Blood Marrow Transplant 2012; 18: 1509–1516.
Seibel NL, Schwartz C, Arrieta A, Flynn P, Shad A, Albano E et al. Safety, tolerability, and pharmacokinetics of Micafungin (FK463) in febrile neutropenic pediatric patients. Antimicrob Agents Chemother 2005; 49: 3317–3324.
Sawada A, Sakata N, Higuchi B, Takeshita Y, Ishihara T, Sakata A et al. [Comparison of micafungin and fosfluconazole as prophylaxis for invasive fungal infection during neutropenia in children undergoing chemotherapy and hematopoietic stem cell transplantation]. Rinsho Ketsueki 2009; 50: 1692–1699.
Hiramatsu Y, Maeda Y, Fujii N, Saito T, Nawa Y, Hara M et al. Use of micafungin versus fluconazole for antifungal prophylaxis in neutropenic patients receiving hematopoietic stem cell transplantation. Int J Hematol 2008; 88: 588–595.
Grigull L, Kuehlke O, Beilken A, Sander A, Linderkamp C, Schmid H et al. Intravenous and oral sequential itraconazole antifungal prophylaxis in paediatric stem cell transplantation recipients: a pilot study for evaluation of safety and efficacy. Pediatr Transplant 2007; 11: 261–266.
McCoy D, Depestel DD, Carver PL . Primary antifungal prophylaxis in adult hematopoietic stem cell transplant recipients: current therapeutic concepts. Pharmacotherapy 2009; 29: 1306–1325.
Joseph JM, Jain R, Danziger LH . Micafungin: a new echinocandin antifungal. Pharmacotherapy 2007; 27: 53–67.
Acknowledgements
This study was coordinated by the Clinical Research Coordination Center, National Cancer Center, Korea, using the web-based clinical research management platform (Velos).
AUTHOR CONTRIBUTIONS
HJP designed the study, collected the data, performed analysis and reviewed the manuscript; MP performed the analysis and wrote the paper; JJS designed the study and reviewed the manuscript; MH collected the data and performed the analysis; BHN performed the anlaysis; KNK, HJI, JWL, N-GC, BC, H-KK, KHY, HHK, HJK, HYS, HSA, YTL, HK, CJL, JOH contributed to data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Park, H., Park, M., Han, M. et al. Efficacy and safety of micafungin for the prophylaxis of invasive fungal infection during neutropenia in children and adolescents undergoing allogeneic hematopoietic SCT. Bone Marrow Transplant 49, 1212–1216 (2014). https://doi.org/10.1038/bmt.2014.136
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2014.136
This article is cited by
-
Micafungin prophylaxis for acute leukemia patients undergoing induction chemotherapy
BMC Cancer (2019)
-
Foiling fungal disease post hematopoietic cell transplant: review of prophylactic strategies
Bone Marrow Transplantation (2018)
-
Comparison of Efficacy and Safety of Caspofungin Versus Micafungin in Pediatric Allogeneic Stem Cell Transplant Recipients: A Retrospective Analysis
Advances in Therapy (2017)
-
Micafungin: A Review in the Prophylaxis and Treatment of Invasive Candida Infections in Paediatric Patients
Pediatric Drugs (2017)