Abstract
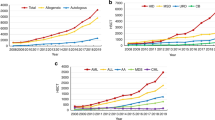
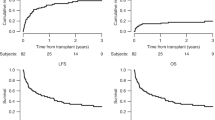
For adults with acute leukemia, it is important to know whether the therapeutic schemes initially planned were actually implemented. The European Group for Blood and Marrow transplantation Acute Leukemia Working Party prospectively followed 695 consecutive patients who were registered at the time of HLA typing. Of 304 patients with an available matched sibling donor (MSD), SCT was planned in 264, chemotherapy in 33 and autografting in 7. For the rest, an unrelated donor (UD) search was initiated in 198. Among these, 117 were transplanted, 114 received chemotherapy and 77 underwent autografting. Probabilities of receiving a planned treatment were 60 and 65% at 1 and 2 years, respectively. Patients scheduled to receive MSD SCT had an 82% probability, whereas those scheduled to undergo UD SCT had a 57% probability, of receiving their transplant at 1 year. The only factor associated with a lower probability of MSD SCT in first remission was delayed HLA typing (HR=0.82; P=0.03). One year after enrollment, 40% of patients did not follow their initial treatment plan. Because OS was 50% only at 3 years and only 57% of the patients without a MSD underwent SCT, this suggests room for improvement in outcomes for adults with acute leukemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Passweg JR, Baldomero H, Gratwohl A, Bregni M, Cesaro S, Dreger P et al. The EBMT activity survey: 1990-2010. Bone Marrow Transplant 2012; 47: 906–923.
Gratwohl A . Cord blood meets its match. Lancet Oncol 2011; 12: 1177–1178.
Baldomero H, Gratwohl M, Gratwohl A, Tichelli A, Niederwieser D, Madrigal A et al. The EBMT activity survey 2009: trends over the past 5 years. Bone Marrow Transplant 2011; 46: 485–501.
Ljungman P, Bregni M, Brune M, Cornelissen J, de Witte T, Dini G et al. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe 2009. Bone Marrow Transplant 2010; 45: 219–234.
Gratwohl A, Baldomero H . European survey on clinical use of cord blood for hematopoietic and non-hematopoietic indications. Transfus Apher Sci 2010; 42: 265–275.
Vellenga E, van Putten W, Ossenkoppele GJ, Verdonck LF, Theobald M, Cornelissen JJ et al. Autologous peripheral blood stem cell transplantation for acute myeloid leukemia. Blood 2011; 118: 6037–6042.
Suciu S . on behalf of the AMLcgoE. Metaanalysis of randomised trials comparing autologous BMT (ABMT) vs chemotherapy (CT) or ABMT vs no further treatment (NFT) as post remission treatment in adult AML patients. Bone Marrow Transplant 1998; 21: 43a.
Cornelissen JJ, Gratwohl A, Schlenk RF, Sierra J, Bornhauser M, Juliusson G et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol 2012; 9: 579–590.
Hehlmann R, Grimwade D, Simonsson B, Apperley J, Baccarani M, Barbui T et al. The European LeukemiaNet: achievements and perspectives. Haematologica 2011; 96: 156–162.
Fine JPG, R.J . A proportional hazards model for subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509.
Ruggeri A, Rocha V, Masson E, Labopin M, Cunha R, Absi L et al. Impact of donor specific anti-HLA antibodies on graft failure and survival after reduced intensity conditioning-unrelated cord blood transplantation. A Eurocord, Societe Francophone d'Histocompatibilite et d'Immunogenetique (SFHI) and Societe Francaise de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC) analysis. Haematologica 2013; 98: 1154–1160.
Scaradavou A, Brunstein CG, Eapen M, Le-Rademacher J, Barker JN, Chao N et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood 2013; 121: 752–758.
Bolanos-Meade J, Fuchs EJ, Luznik L, Lanzkron SM, Gamper CJ, Jones RJ et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood 2012; 120: 4285–4291.
Huang XJ, Zhu HH, Chang YJ, Xu LP, Liu DH, Zhang XH et al. The superiority of haploidentical related stem cell transplantation over chemotherapy alone as postremission treatment for patients with intermediate- or high-risk acute myeloid leukemia in first complete remission. Blood 2012; 119: 5584–5590.
Cassileth PA, Harrington DP, Appelbaum FR, Lazarus HM, Rowe JM, Paietta E et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. N Engl J Med 1998; 339: 1649–1656.
Harousseau JL, Cahn JY, Pignon B, Witz F, Milpied N, Delain M et al. Comparison of autologous bone marrow transplantation and intensive chemotherapy as postremission therapy in adult acute myeloid leukemia. The Groupe Ouest Est Leucemies Aigues Myeloblastiques (GOELAM). Blood 1997; 90: 2978–2986.
Zittoun RA, Mandelli F, Willemze R, de Witte T, Labar B, Resegotti L et al. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto (GIMEMA) Leukemia Cooperative Groups. N Engl J Med 1995; 332: 217–223.
Giebel S, Labopin M, Ehninger G, Beelen D, Blaise D, Ganser A et al. Association of human development index with rates and outcomes of hematopoietic stem cell transplantation for patients with acute leukemia. Blood 2010; 116: 122–128.
Velardi A . Haplo-BMT: which approach? Blood 2013; 121: 719–720.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Labopin, M., Gorin, NC., Polge, E. et al. A prospective registration study to determine feasibility of hematopoietic SCT in adults with acute leukemia: planning, expectations and reality. Bone Marrow Transplant 49, 376–381 (2014). https://doi.org/10.1038/bmt.2013.178
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.178
Keywords
This article is cited by
-
Access to alternative donor hematopoietic search and transplantation for acute leukemia in different macro-regions of Italy. A GITMO/IBMDR study
Bone Marrow Transplantation (2018)
-
Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia
Leukemia (2015)
-
Allogeneic hematopoietic cell transplantation for acute myeloid leukemia during first complete remission: a clinical perspective
International Journal of Hematology (2015)
-
Should persons with acute myeloid leukemia have a transplant in first remission?
Leukemia (2014)