Abstract
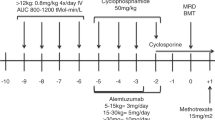
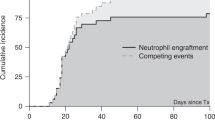
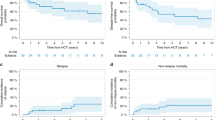
This retrospective national study compared the use of alemtuzumab-based conditioning regimens for hematopoietic SCT (HSCT) in acquired severe aplastic anemia with antithymocyte globulin (ATG)-based regimens. One hundred patients received alemtuzumab and 55 ATG-based regimens. A matched sibling donor (MSD) was used in 87 (56%), matched unrelated donor (MUD) in 60 (39%) and other related or mismatched unrelated donor (UD) in 8 (5%) patients. Engraftment failure occurred in 9% of the alemtuzumab group and 11% of the ATG group. Five-year OS was 90% for the alemtuzumab and 79% for the ATG groups, P=0.11. For UD HSCT, OS of patients was better when using alemtuzumab (88%) compared with ATG (57%), P=0.026, although smaller numbers of patients received ATG. Similar outcomes for MSD HSCT using alemtuzumab or ATG were seen (91% vs 85%, respectively, P=0.562). A lower risk of chronic GVHD (cGVHD) was observed in the alemtuzumab group (11% vs 26%, P=0.031). On multivariate analysis, use of BM as stem cell source was associated with better OS and EFS, and less acute and cGVHD; young age was associated with better EFS and lower risk of graft failure. This large study confirms successful avoidance of irradiation in the conditioning regimens for MUD HSCT patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Armand P, Antin JH . Allogeneic stem cell transplantation for aplastic anemia. Biol Blood Marrow Transplant 2007; 13: 505–516.
Bacigalupo A, Brand R, Oneto R, Bruno B, Socie G, Passweg J et al. Treatment of acquired severe aplastic anemia: bone marrow transplantation compared with immunosuppressive therapy—The European Group for Blood and Marrow Transplantation experience. Semin Hematology 2000; 37: 69–80.
Storb R, Etzioni R, Anasetti C, Appelbaum FR, Buckner CD, Bensinger W et al. Cyclophosphamide combined with antithymocyte globulin in preparation for allogeneic marrow transplants in patients with aplastic anemia. Blood 1994; 84: 941–949.
Storb R, Leisenring W, Anasetti C, Appelbaum FR, Buckner CD, Bensinger WI et al. Long-term follow-up of allogeneic marrow transplants in patients with aplastic anemia conditioned by cyclophosphamide combined with antithymocyte globulin. Blood 1997; 89: 3890–3891.
Champlin RE, Perez W, Passweg J, Klein J, Camitta B, Gluckman E et al. Bone marrow transplantation for severe aplastic anemia: a randomized controlled study of conditioning regimens. Blood 2007; 109: 4582–4585.
Locatelli F, Bruno B, Zecca M, Van-Lint MT, McCann S, Arcese W et al. Cyclosporin A and short-term methotrexate versus cyclosporin A as graft versus host disease prophylaxis in patients with severe aplastic anemia given allogeneic bone marrow transplantation from an HLA-identical sibling: results of a GITMO/EBMT randomized trial. Blood 2000; 96: 1690–1697.
Maury S, Bacigalupo A, Anderlini P, Aljurf M, Marsh J, Socie G et alon behalf of the Severe Aplastic Anaemia Working Party, European Group for Blood and marrow Transplantation. Improving outcome of patients older than 30 years receiving HLA-identical sibling HSCT for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica 2009; 94: 1312–1315.
Schrezenmeier H, Passweg J, Marsh JCW, Bacigalupo A, Bredeson CN, Bullorsky E et al. Worse outcome and more chronic GVHD with peripheral blood progenitor cells than bone marrow in HLA-matched sibling donor transplants for young patients with severe acquired aplastic anemia. Blood 2007; 110: 1397–1400.
Bacigalupo A, Socié G, Schrezenmeier H, Tichelli A, Locasciulli A, Fuehrer M et alfor the Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation (WPSAA-EBMT). Bone marrow versus peripheral blood sibling transplants in acquired aplastic anemia: survival advantage for marrow in all age groups. Haematologica 2012; 97: 1142–1148.
Maury S, Balere-Appert M, Chir Z, Boiron J, Galambrun C, Yakouben K et alfor the French Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC). Unrelated stem cell transplantation for severe acquired aplastic anemia: improved outcome in the era of high-resolution HLA matching between donor and recipient. Haematologica 2007; 92: 589–596.
Bacigalupo A, Locatelli F, Dini G, Marsh J, Pession A, Socie G et alfor the Severe Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation, (SAA WP-EBMT). Fludarabine, cyclophosphamide and anti-thymocyte globulin for alternative donor transplants in acquired severe aplastic anemia: a report from the EBMT-SAA Working Party. Bone Marrow Transplant 2005; 36: 947–950.
Bacigalupo A, Socie G, Lanino E, Prete A, Locatelli F, Locasciulli A et alfor the Severe Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation. Fludarabine, cyclophosphamide, antithymocyte globulin, with or without low dose total body irradiation, for alternative donor transplants, in acquired severe aplastic anemia: a retrospective study from the EBMT-SAA working party. Haematologica 2010; 95: 976–982.
Deeg HJ, Amylon ID, Harris RE, Collins R, Beatty PG, Feig S et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant 2001; 7: 208–215.
Kojima S, Matsuyama T, Kato S, Kigasawa H, Kobayashi R, Kikuta A et al. Outcome of 154 patients with severe aplastic anemia who received transplants from unrelated donors: the Japan Marrow Donor Program. Blood 2002; 100: 799–803.
Kennedy-Nasser A, Leuknig K, Mahiajan A, Weiss H, Arce J, Gottschalk S et al. Comparable outcomes of matched-related and alternative donor stem cell transplantation for pediatric severe aplastic anemia. Biol Blood Marrow Transplant 2006; 12: 1277–1284.
Yagasaki H, Takahashi Y, Hama A, Kudo K, Nishio N, Muramatsu H et al. Comparison of matched-sibling donor BMT and unrelated donor BMT in children and adolescent with acquired severe aplastic anaemia. Bone Marrow Transplant 2010; 45: 1508–1513.
Deeg H, O'Donnell M, Tolar j, Agarwal R, Harris R, Feig S et al. Optimization of conditioning for marrow transplantation from unrelated donors for patients with aplastic anemia after failure of immunosuppressive therapy. Blood 2006; 108: 1485–1491.
Konopacki J, Porcher R, Robin M, Bieri S, Cayuela JM, Larghero J et al. Long-term follow up after allogeneic stem cell transplantation in patients with severe aplastic anemia after cyclophosphamide plus antithymocyte globulin conditioning. Haematologica 2012; 97: 710–716.
Passweg JR, Aljurf M . Treatment and hematopoietic SCT in aplastic anemia. Bone Marrow Transplant 2013; 48: 161.
Marsh JCW, Ball SE, Cavenagh J, Darbyshire P, Dokal I, Gordon-Smith EC et alfor the British Committee for Standards in Haematology (BCSH). Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol 2009; 147: 43–70.
Marsh JC, Gupta V, Lim Z, Ho AY, Ireland R, Hayden J et al. Alemtuzumab with fludarabine and cyclophosphamide reduces chronic graft versus host disease after allogeneic stem cell transplantation for acquired aplastic anemia. Blood 2011; 118: 2351–2357.
Camitta BM, Thomas ED, Nathan DG, Santos G, Gordon-Smith EC, Gale RP et al. Severe aplastic anemia: a prospective study of the effect of early marrow transplantation on acute mortality. Blood 1976; 48: 63–70.
Incidence of aplastic anemia: the relevance of diagnostic criteria. By the International Agranulocytosis and Aplastic Anemia Study. Blood 1987; 70: 1718–1721.
Bacigalupo A, Hows J, Gluckman E, Nissen C, Marsh J, Van Lint MT et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol 1988; 70: 177–182.
Lee J-H, Choi S-J, Lee J-H, Lee YS, Seol M, Ryu SG et al. Non-total body irradiation containing preparative regimen in alternative donor bone marrow transplantation for severe aplastic anemia. Bone Marrow Transplant 2005; 35: 755–761.
Kang HJ, Shin HY, Park JE, Chung NG, Cho B, Kim HK et al. Successful engraftment with fludarabine, cyclophosphamide and thymoglobulin conditioning regimen in unrelated transplantation for severe aplastic anemia: a phase II prospective multicenter study. Biol Blood Marrow Transplant 2010; 11: 1582–1588.
Deeg HJ, Leisenring W, Storb R, Nims J, Flowers MED, Witherspoon RP et al. Long-term outcome after marrow transplantation for severe aplastic anemia. Blood 1998; 91: 3637–3645.
Ades L, Mary JY, Robin M, Ferry C, Porcher R, Esperou H et al. Long-term outcome after bone marrow transplantation for severe aplastic anemia. Blood 103: 2490–2497.
Socié G, Salooja N, Cohen A, Rovelli A, Carreras E, Locasciulli A et al. Late Effects Working Party of the European Study Group for Blood and Marrow Transplantation. Non-malignant late effects after allogeneic stem cell transplantation. Blood 2003; 101: 3373–3385.
Sanders JE, Hoffmeister PA, Woolfrey AE, Carpenter PA, Storer BE, Storb RF et al. Thyroid function following hematopoeitic cell transplantation in children: 30 years’ experience. Blood 2009; 113: 306–308.
Sanders JE, Woolfry AE, Carpenter PA, Storer BE, Hoffmeister PA, Deeg HJ et al. Late effects among pediatric patients follwed for nearly 4 decades after transplantation for severe aplastic anemia. Blood 2011; 18: 1421–1428.
Samarasinghe S, Steward C, Hiwarkar P, Saif MA, Hough R, Webb D et al. Excellent outcomes of matched unrelated donor transplantation in paediatric aplastic aanemia following failure with immunosuppressive therapy: a United Kingdom multicentre retrospective experience. Br J Haematol 2012; 157: 339–346.
Kosaka Y, Yagasaki H, Sano K, Kobayashi R, Ayukawa H, Kaneko T et al. Prospective multicenter trial comparing repeated immunosuppressive therapy with stem-cell transplantation from an alternative donor as second-line treatment for children with severe and very severe aplastic anemia. Blood 2008; 111: 1054–1059.
Sangiolo D, Storb R, Deeg HJ, Flowers MED, Martin PJ, Sandmaier BM et al. Outcome of allogeneic hematopoietic cell transplantation from HLA-identical siblings for severe aplastic anemia in patients over 40 years of age. Biol Blood Marrow Transplant 2010; 16: 1411–1418.
Gupta V, Eapen M, Bajorunaite R, Carreras J, Aljurf M, Gale RP et alon behalf of the Writing Committee. Impact of age on outcomes after transplantation for acquired aplastic anemia using HLA-identical sibling donors. Haematologica 2010; 95: 2119–2125.
Eapen M, Le-Rademacher J, Antin J, Champlin RE, Carreras J, Fay J et al. Effect of stem cell source on outcomes after adult unrelated donor transplantation in severe aplastic anemia. Blood 2011; 118: 2618–2621.
Acknowledgements
We would like to thank the data managers and transplant physicians at participating centers for providing data: Dr MBC Koh, data manager S Kukkapalli, St George’s Hospital, London; Dr J Snowden, data managers B Holt and L Scott, Sheffield teaching Hospitals, Sheffield; Dr AJ Vora, data manger J Williams, Sheffield Children’s Hospital, Sheffield; Dr RT Wynn, data manager M Coussons, Royal Manchester Children’s Hospital, Manchester; Professor N Russell, data manager P Nelson, Nottingham University Hospital, Nottingham; Dr B Gibson, Yorkhill Children’s Hospital, data manager G Stewart, Glasgow; Dr M Gilleece, data managers S Hardaker, R Goodall, St James University Hospital, Leeds; Dr D Milligan, data manager W Clay, Heartlands Hospital, Birmingham; Dr P Veys, Great Ormond Street Hospital, London; Dr S Samarasinghe, Great North Children’s Hospital, Newcastle; Dr M McMullin, data manager S Piggott, Belfast City Hospital, Belfast; Prof G Jackson, data manager L McNally, Royal Victoria Infirmary, Newcastle; Dr K Orchard, data manager C Hurlock, Southampton General Hospital, Southampton; Dr A Hunter, data manager R Lewin, Leicester Royal Infirmary, Leicester; Prof C Craddock, data manager J Ward, Queen Elizabeth Hospital, Birmingham; Dr A O’Meara, data managers H Kerrigan and C Cawley, Our Lady’s Hospital for Sick Children, Dublin; Prof J Apperley, data manager F O’Boyle, Hammersmith Hospital, London; Dr K Wilson, data manager S Nicholas, University Hospital of Wales, Cardiff; Dr A Bloor, data manager T Dalton, Christie Hospital, Manchester; Dr P Johnson, data manager A Robertson, Western General Hospital, Edinburgh; Dr S Lawson, data manager J Rogers, Birmingham Children’s Hospital, Birmingham; Dr J dela Fuente, data manager F O’Boyle, St Mary’s Hospital, London; Dr K Thomson, University College Hospital, London.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
JCM held a consultancy with Genzyme from May 2008 to May 2009 and from June 2009 to July 2009. The other authors declare no competing financial interests.
Additional information
Authors contribution
JCM, RMP, GC, ZL, GJM, AP contributed to the design of the study. JCM drafted the manuscript. KK and JP were responsible for coordination of data acquisition. JP, KK, MBCK, JAS, AJV, RTW, NR, BG, MG, DM, PV, SS, MM and GC contributed patient data to the study. RMP performed statistical analysis of the data. All authors were responsible for critical review and revision of the manuscript.
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
Marsh, J., Pearce, R., Koh, M. et al. Retrospective study of alemtuzumab vs ATG-based conditioning without irradiation for unrelated and matched sibling donor transplants in acquired severe aplastic anemia: a study from the British Society for Blood and Marrow Transplantation. Bone Marrow Transplant 49, 42–48 (2014). https://doi.org/10.1038/bmt.2013.115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.115
Keywords
This article is cited by
-
Allogeneic bone marrow transplantation for aplastic anemia
International Journal of Hematology (2024)
-
Efficacy and Safety of Eltrombopag for Aplastic Anemia: A Systematic Review and Meta-analysis
Clinical Drug Investigation (2019)
-
Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019
Bone Marrow Transplantation (2019)
-
Acquired aplastic anemia in Korean children: treatment guidelines from the Bone Marrow Failure Committee of the Korean Society of Pediatric Hematology Oncology
International Journal of Hematology (2016)
-
Indications for allo- and auto-SCT for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2015
Bone Marrow Transplantation (2015)