Abstract
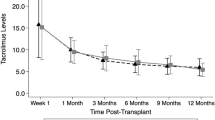
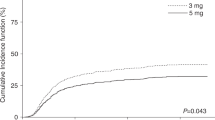
Tacrolimus is routinely administered for GVHD prophylaxis as a 24-h continuous infusion that requires a dedicated i.v. line and thus becomes logistically difficult to administer, especially in young pediatric patients. We investigated the safety and efficacy of twice daily bolus infusions of i.v. tacrolimus in 33 children undergoing hematopoietic stem cell transplantation (HSCT) at our institution. Tacrolimus was started at an initial dose of 0.015 mg/kg i.v. bolus administered as a 2-h infusion and then given at every 12 h to maintain a trough drug level between 5–15 ng/mL. Patients also received short-course MTX (66%) or mycophenolate mofetil (34%) in combination with tacrolimus. No acute infusional toxicities were observed with bolus infusions of i.v. tacrolimus. Nephrotoxicity occurred in 14/33 (42%) patients and 48% developed hypertension (HT). Almost all (94%) patients required magnesium supplements to maintain magnesium (Mg) levels ⩾1.5 mg/dL. In all, 3 (9%) patients developed severe sinusoidal obstruction syndrome (SOS). One patient developed posterior reversible leuko-encephalopathy syndrome (PRES) and one additional patient had tremors. The prevelance of these side-effects was similar to those reported for continuous i.v. administration. In all, 28% of the evaluable patients developed acute GVHD⩾grade II, though the incidence of severe (grade III–IV) GVHD was only 7%. These results suggest that intermittent bolus i.v. tacrolimus administration is a safe and effective method of GVHD prophylaxis in children.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Tanaka H, Kuroda A, Marusawa H, Hashimoto M, Hatanaka H, Kino T et al. Physicochemical properties of FK-506, a novel immunosuppressant isolated from Streptomyces tsukubaensis. Transplant Proc 1987; 19(5 Suppl 6): 11–16.
Bierer BE, Hollander G, Fruman D, Burakoff SJ . Cyclosporin A and FK506: molecular mechanisms of immunosuppression and probes for transplantation biology. Curr Opin Immunol 1993; 5: 763–773.
Jacobson P, Uberti J, Davis W, Ratanatharathorn V . Tacrolimus: a new agent for the prevention of graft-versus-host disease in hematopoietic stem cell transplantation. Bone Marrow Transplant 1998; 22: 217–225.
Hiraoka A, Ohashi Y, Okamoto S, Moriyama Y, Nagao T, Kodera Y et al. Phase III study comparing tacrolimus (FK506) with cyclosporine for graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation. Bone Marrow Transplant 2001; 28: 181–185.
Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood 2000; 96: 2062–2068.
Boswell GW, Bekersky I, Fay J, Wingard J, Antin J, Weisdorf D et al. Tacrolimus pharmacokinetics in BMT patients. Bone Marrow Transplant 1998; 21: 23–28.
Yanik G, Levine JE, Ratanatharathorn V, Dunn R, Ferrara J, Hutchinson RJ . Tacrolimus (FK506) and methotrexate as prophylaxis for acute graft-versus-host disease in pediatric allogeneic stem cell transplantation. Bone Marrow Transplant 2000; 26: 161–167.
Yanagisawa R, Katsuyama Y, Shigemura T, Saito S, Tanaka M, Nakazawa Y et al. Engraftment syndrome, but not acute GVHD, younger age, CYP3A5 or MDR1 polymorphisms, increases tacrolimus clearance in pediatric hematopoietic SCT. Bone Marrow Transplant 2011; 46: 90–97.
Wingard JR, Nash RA, Przepiorka D, Klein JL, Weisdorf DJ, Fay JW et al. Relationship of tacrolimus (FK506) whole blood concentrations and efficacy and safety after HLA-identical sibling bone marrow transplantation. Biol Blood Marrow Transplant 1998; 4: 157–163.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Wong R, Beguelin GZ, de Lima M, Giralt SA, Hosing C, Ippoliti C et al. Tacrolimus-associated posterior reversible encephalopathy syndrome after allogeneic haematopoietic stem cell transplantation. Br J Haematol 2003; 122: 128–134.
Ho VT, Cutler C, Carter S, Martin P, Adams R, Horowitz M et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2005; 11: 571–575.
McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 1993; 118: 255–267.
Fay JW, Wingard JR, Antin JH, Collins RH, Pineiro LA, Blazar BR et al. FK506 (Tacrolimus) monotherapy for prevention of graft-versus-host disease after histocompatible sibling allogenic bone marrow transplantation. Blood 1996; 87: 3514–3519.
Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood 1998; 92: 2303–2314.
Navaneethan SD, Sankarasubbaiyan S, Gross MD, Jeevanantham V, Monk RD . Tacrolimus-associated hypomagnesemia in renal transplant recipients. Transplant Proc 2006; 38: 1320–1322.
Pirsch JD, Miller J, Deierhoi MH, Vincenti F, Filo RS . A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 Kidney Transplant Study Group. Transplantation 1997; 63: 977–983.
Mayer AD, Dmitrewski J, Squifflet JP, Besse T, Grabensee B, Klein B et al. Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection: a report of the European Tacrolimus Multicenter Renal Study Group. Transplantation 1997; 64: 436–443.
Mueller AR, Platz KP, Christe W, Bechstein WO, Blumhardt G, Neuhaus P . Severe neurotoxicity after liver transplantation: association between FK 506 therapy and hepatitis C virus disease. Transplant Proc 1994; 26: 3131–3132.
Wijdicks EF, Wiesner RH, Dahlke LJ, Krom RA . FK506-induced neurotoxicity in liver transplantation. Ann Neurol 1994; 35: 498–501.
Osunkwo I, Bessmertny O, Harrison L, Cheung YK, Van de Ven C, del Toro G et al. A pilot study of tacrolimus and mycophenolate mofetil graft-versus-host disease prophylaxis in childhood and adolescent allogeneic stem cell transplant recipients. Biol Blood Marrow Transplant 2004; 10: 246–258.
Shaw PJ, Kan F, Woo Ahn K, Spellman SR, Aljurf M, Ayas M et al. Outcomes of pediatric bone marrow transplantation for leukemia and myelodysplasia using matched sibling, mismatched related, or matched unrelated donors. Blood 2010; 116: 4007–4015.
Weisdorf D, Hakke R, Blazar B, Miller W, McGlave P, Ramsay N et al. Risk factors for acute graft-versus-host disease in histocompatible donor bone marrow transplantation. Transplantation 1991; 51: 1197–1203.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Skeens, M., Pai, V., Garee, A. et al. Twice daily i.v. bolus tacrolimus infusion for GVHD prophylaxis in children undergoing stem cell transplantation. Bone Marrow Transplant 47, 1415–1418 (2012). https://doi.org/10.1038/bmt.2012.59
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2012.59