Abstract
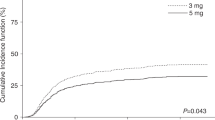
Post-transplant cyclophosphamide (PTCy) combined with tacrolimus (TAC) as graft-versus-host disease (GvHD) prophylaxis post-hematopoietic cell transplantation (HCT) is safe and effective. Optimal serum levels of TAC in this combination remain undetermined. We hypothesized that TAC at initial steady state (TISS) of <10 ng/mL could promote optimal transplant outcomes and prevent TAC-associated toxicities. We retrospectively analyzed a consecutive case series of 210 patients who received PTCy/TAC-based prophylaxis post-HCT from 1/2013-6/2018. Patients received HCT from haploidentical (n = 172) or mismatched donors (n = 38), and flat dose (FD) or weight-based dose (WBD) TAC. Twenty-four-month overall survival (OS), disease free survival (DFS), and relapse rate (RR) were 61%, 56%, and 22%, respectively, in TISS < 10 ng/mL cohort (n = 176), and 50%, 43%, and 35%, respectively, in TISS ≥ 10 ng/mL cohort (n = 34) (OS, P = 0.71; DFS, P = 0.097; RR, P = 0.031). OS, DFS, RR, non-relapse mortality, acute GvHD grade II–IV, grade III–IV or chronic GvHD by TISS were similar in multivariable analysis. TISS ≥ 10 ng/mL conferred increased risk of viral infection (P = 0.003). More patients receiving FD vs. WBD had TISS < 10 ng/mL (P = 0.001). Overall, TISS < 10 ng/mL early post HCT conferred similar survival outcomes and lowered risk of viral infection and toxicities compared to TISS ≥ 10 ng/mL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood 2015;126:1033–40.
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–50.
Al Malki MM, Tsai N-C, Palmer J, Mokhtari S, Cao T, Ali H, et al. A Phase II trial of post-transplant cyclophosphamide as graft-versus-host disease prophylaxis in HLA-mismatched unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2020;26:S188 3, Supplement.
Shaw BE, Burns LJ, Logan B, Jimenez-Jimenez AM, Khimani F, Shaffer BC, et al. Transplantation using bone marrow from a (very) HLA mismatched unrelated donor in the setting of post-transplant cyclophosphamide is feasible and expands access to underserved minorities. Biol Blood Marrow Transplant. 2020;26:S283–S4. 3, Supplement.
Bashey A, Zhang MJ, McCurdy SR, St Martin A, Argall T, Anasetti C, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35:3002–9.
Carnevale-Schianca F, Caravelli D, Gallo S, Coha V, D’Ambrosio L, Vassallo E, et al. Post-transplant cyclophosphamide and tacrolimus-mycophenolate mofetil combination prevents graft-versus-host disease in allogeneic peripheral blood hematopoietic cell transplantation from HLA-matched donors. Biol Blood Marrow Transplant. 2017;23:459–66.
Ruggeri A, Labopin M, Bacigalupo A, Afanasyev B, Cornelissen JJ, Elmaagacli A, et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol Oncol. 2018;11:40.
Battipaglia G, Labopin M, Kröger N, Vitek A, Afanasyev B, Hilgendorf I, et al. Posttransplant cyclophosphamide vs antithymocyte globulin in HLA-mismatched unrelated donor transplantation. Blood 2019;134:892–9.
Shayani S, Palmer J, Stiller T, Liu X, Thomas SH, Khuu T, et al. Thrombotic microangiopathy associated with sirolimus level after allogeneic hematopoietic cell transplantation with tacrolimus/sirolimus-based graft-versus-host disease prophylaxis. Biol Blood Marrow Transpl. 2013;19:298–304.
Hodnett P, Coyle J, O’Regan K, Maher MM, Fanning N. PRES (posterior reversible encephalopathy syndrome), a rare complication of tacrolimus therapy. Emerg Radiol. 2009;16:493–6.
Przepiorka D, Nash RA, Wingard JR, Zhu J, Maher RM, Fitzsimmons WE, et al. Relationship of tacrolimus whole blood levels to efficacy and safety outcomes after unrelated donor marrow transplantation. Biol Blood Marrow Transplant. 1999;5:94–7.
Cutler C, Kim HT, Hochberg E, Ho V, Alyea E, Lee SJ, et al. Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2004;10:328–36.
Nakamura R, Rodriguez R, Nademanee A, Palmer J, Senitzer D, Snyder D, et al. The use of sirolimus combined with Tacrolimus and low-dose methotrexate is effective in preventing graft-versus-host disease after unrelated donor hematopoietic stem cell transplantation. Blood 2006;108:2866.
Khaled SK, Palmer JM, Herzog J, Stiller T, Tsai NC, Senitzer D, et al. Influence of absorption, distribution, metabolism, and excretion genomic variants on tacrolimus/sirolimus blood levels and graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:268–76.
Wingard JR, Nash RA, Przepiorka D, Klein JL, Weisdorf DJ, Fay JW, et al. Relationship of tacrolimus (FK506) whole blood concentrations and efficacy and safety after HLA-identical sibling bone marrow transplantation. Biol Blood Marrow Transplant. 1998;4:157–63.
Przepiorka D, Devine S, Fay J, Uberti J, Wingard J. Practical considerations in the use of tacrolimus for allogeneic marrow transplantation. Bone Marrow Transplant. 1999;24:1053–6.
Cutler C, Logan B, Nakamura R, Johnston L, Choi S, Porter D, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood 2014;124:1372–7.
Butts AR, Brown VT, McBride LD, Bolaños-Meade J, Bryk AW. Factors associated with optimized tacrolimus dosing in hematopoietic stem cell transplantation. J Oncol Pharm Pract. 2016;22:275–83.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–17.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8.
Kasamon YL, Luznik L, Leffell MS, Kowalski J, Tsai HL, Bolaños-Meade J, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16:482–9.
Ganetsky A, Shah A, Miano TA, Hwang WT, He J, Loren AW, et al. Higher tacrolimus concentrations early after transplant reduce the risk of acute GvHD in reduced-intensity allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51:568–72.
Cristiano S, Giorgia F, Sec Julie H, Marco R, Estefania Nova L, Mitalee S, et al. Impact of immunosuppressive drugs on the therapeutic efficacy of ex vivo expanded human regulatory T cells. Haematologica 2016;101:91–100.
García Cadenas I, Valcarcel D, Martino R, Piñana JL, Barba P, Novelli S, et al. Impact of cyclosporine levels on the development of acute graft versus host disease after reduced intensity conditioning allogeneic stem cell transplantation. Mediators Inflamm. 2014;2014:620682.
de Kort EA, de Lil HS, Bremmers MEJ, van Groningen LFJ, Blijlevens NMA, Huls G, et al. Cyclosporine A trough concentrations are associated with acute GvHD after non-myeloablative allogeneic hematopoietic cell transplantation. PLoS One. 2019;14:e0213913–e.
Malard F, Szydlo RM, Brissot E, Chevallier P, Guillaume T, Delaunay J, et al. Impact of cyclosporine-A concentration on the incidence of severe acute graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:28–34.
Yee GC, Self SG, McGuire TR, Carlin J, Sanders JE, Deeg HJ. Serum cyclosporine concentration and risk of acute graft-versus-host disease after allogeneic marrow transplantation. N. Engl J Med. 1988;319:65–70.
Martin P, Bleyzac N, Souillet G, Galambrun C, Bertrand Y, Maire PH, et al. Relationship between CsA trough blood concentration and severity of acute graft-versus-host disease after paediatric stem cell transplantation from matched-sibling or unrelated donors. Bone Marrow Transplant. 2003;32:777–84.
Sharma N, Zhao Q, Ni B, Elder P, Puto M, Benson DM, et al. Effect of early post-transplantation tacrolimus concentration on the risk of acute graft-versus-host disease in allogenic stem cell transplantation. Cancers 2021;13:613.
Acknowledgements
Research reported in this publication included work performed in the Biostatistics and Mathematical Modeling Core supported by the National Cancer Institutes of Health under grant number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
JMY and MMA designed the study, collected and analyzed the data, and prepared the manuscript; DY performed statistical analysis, interpreted data, and prepared the manuscript; MCC interpreted data and prepared the manuscript; SO, TC, HA, SA, IA, AA, IA, AS, VP, KS, AS, GM, SJF, and RN interpreted data and provided critical feedback of the manuscript; and all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Research reported in this publication included work performed in the Biostatistics and Mathematical Modeling Core supported by the National Cancer Institutes of Health under grant number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no relevant conflicts of interest to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yao, J.M., Yang, D., Clark, M.C. et al. Tacrolimus initial steady state level in post-transplant cyclophosphamide-based GvHD prophylaxis regimens. Bone Marrow Transplant 57, 232–242 (2022). https://doi.org/10.1038/s41409-021-01528-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-021-01528-y