Abstract
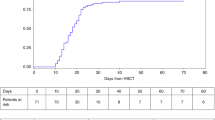
We report long-term outcomes of 329 childhood severe aplastic anemia (SAA) patients who underwent hematopoietic SCT (HSCT) from an HLA-matched sibling donor in the Japanese Hematopoietic Cell Transplantation Registry. OS and EFS at 10 years were as high as 89.7+/−1.7% and 85.5+/−2.0%, respectively. Five cases of late malignancies (LM) were identified (malignant peripheral nerve sheath tumor, thyroid carcinoma, colon carcinoma, MDS and hepatoblastoma). Cumulative incidence of LM was 0.8% at 10 years and 2.5% at 20 years, respectively, which was lower than that in previous reports. This low incidence is in keeping with the low occurrence of skin cancer in Japanese population and of acute GVHD in our study group. Radiation-containing conditioning was not significantly associated with the incidence of LM after HSCT probably because of absolute low patient number who developed LM in our series. In terms of LM development after HSCT, low-dose TBI in HSCT for SAA to avoid graft rejection, which is commonly used in Japan, might be tolerable in the Japanese population because of its low incidence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Davies JK, Guinan EC . An update on the management of severe idiopathic aplastic anaemia in children. Br J Haematol 2007; 136: 549–564.
Kobayashi R, Yabe H, Hara J, Morimoto A, Tsuchida M, Mugishima et al. Preceding immunosuppressive therapy with anti-thymocyte globulin and ciclosporin increases the incidence of graft rejection in children with aplastic anaemia who underwent allogeneic bone marrow transplantation from HLA-identical siblings. Br J Haematol 2006; 135: 693–696.
Kolb HJ, Socie G, Duell T, Van Lint MT, Tichelli A, Apperley JF et al. Malignant neoplasms in long-term survivors of bone marrow transplantation. Ann Intern Med 1993; 131: 738–744.
Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socie G, Travis LB et al. Solid cancers after bone marrow transplantation. N Engl J Med 1997; 336: 897–904.
Socie G, Henry-Amar M, Bacigalpo A, Hows J, Tichelli A, Ljungman P et al. Malignant tumors occurring after treatment of aplastic anemia. N Engl J Med 1993; 329: 1152–1157.
Ades L, Mary JY, Robin M, Ferry C, Porcher R, Esperou H et al. Long-term outcome after bone marrow transplantation for severe aplastic anemia. Blood 2004; 103: 2490–2497.
Ishimura M, Ohga S, Nagatoshi Y, Okamura J, Tajiri T, Kohashi K et al. Malignant hepatic tumor occurring 10 yrs after histocompatible sibling donor bone marrow transplantation for severe aplastic anemia. Pediatr Transplant 2007; 11: 945–959.
Burroughs LM, Woolfrey AE, Storer BE, Deeg HJ, Flowers MED, Martin PJ et al. Success of allogeneic marrow transplantation for children with severe aplastic anemia. Br J Haematol 2012; 158: 120–128.
Miller DL, Weinstock MA . Nonmelanoma skin cancer in the United States: incidence. J Am Aca Dermatol 1994; 30: 774–778.
Tada M, Miki Y . Malignant skin tumors among dertmatology patients in university hospitals of Japan. J Dermatol 1984; 11: 313–321.
Guardiola P, Socie G, Li X, Ribaud P, Devergie A, Esperou H et al. Acute graft-versus-host disease in patients with Fanconi anemia or acquired aplastic anemia undergoing bone marrow transplantation from HLA-identical sibling donors: risk factors and influence on outcome. Blood 2004; 103: 73–77.
Oh H, Loberiza FR, Zhang M, Ringden O, Akiyama H, Asai T et al. Comparison of graft-versus-host-disease and survival after HLA-identical sibling bone marrow transplantation in ethnic populations. Blood 2005; 105: 1408–1416.
Rizzo JD, Curtis RE, Socié G, Sobocinski KA, Gilbert E, Landgren O et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood 2009; 113: 1175–1183.
Maury S, Bacigalupo A, Anderlini P, Aljurf M, Marsh J, Socié G et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica 2009; 94: 1312–1315.
Bacigalupo A, Socie G, Lanino E, Prete A, Locatelli F, Locasciulli A et al. Fludarabine, cyclophosphamide, antithymocyte globulin, with or without low dose total body irradiation, for alternative donor transplants, in acquired severe aplastic anemia: a retrospective study from the EBMT-SAA working party. Haematologica 2010; 95: 976–982.
Yabe M, Shimizu T, Morimoto T, Koike T, Takakura H, Suganuma E et al. Alternative donor marrow transplantation in children with aplastic anemia using low-dose irradiation and fludarabine-based conditioning. Bone Marrow Transplant 2011; 46: 1148–1150.
Acknowledgements
We thank all participating doctors and patients who were involved in the Japanese Hematopoietic Cell Transplantation Registry.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kikuchi, A., Yabe, H., Kato, K. et al. Long-term outcome of childhood aplastic anemia patients who underwent allogeneic hematopoietic SCT from an HLA-matched sibling donor in Japan. Bone Marrow Transplant 48, 657–660 (2013). https://doi.org/10.1038/bmt.2012.205
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2012.205
Keywords
This article is cited by
-
Recent advances in the diagnosis and treatment of pediatric acquired aplastic anemia
International Journal of Hematology (2024)
-
A New Immunosuppressive Therapy for Very Severe Aplastic Anemia in Children with Autoantibodies
Current Medical Science (2022)
-
Updated Guidelines for the Treatment of Acquired Aplastic Anemia in Children
Current Oncology Reports (2018)
-
Second allogeneic hematopoietic stem cell transplantation in children with severe aplastic anemia
Bone Marrow Transplantation (2015)