Abstract
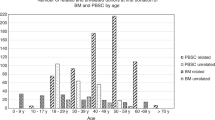
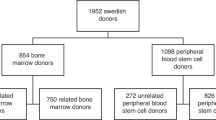
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common RBC enzymatic disorder in humans capable of producing hemolytic events. Recently, concern has been raised about using G6PD-deficienct subjects as hemopoietic stem cell (HSC) donors. In a 10-year period, 101 consecutive HSC donors were submitted to donation procedures for transplantation inside their families in our Center. All donors were tested for G6PD and 19 (19%) turned out to be G6PD-deficient. The donors’ safety and the effectiveness of these transplant outcomes were compared with those of the remaining 82 donors. No difference could be observed in any safety parameter between the two groups. No difference was recorded in donors’ complications rates, in HSC production, in quantity of growth factor required, in Hb early drop or in Hb recovery. No difference was found in transplant outcome. From this retrospective analysis, we conclude that a G6PD-deficient but otherwise healthy volunteer can be selected as a HSC donor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Beutler E . Gene inactivation: the distribution of gene products among populations of cells in heterozygous humans. Cold Spring Harb Symp Quant Biol 1964; 29: 261–271.
Beutler E . Glucose-6-phosphate dehydrogenase deficiency. N Engl J Med 1991; 324: 169–174.
Beutler E . The genetics of glucose-6-phosphate dehydrogenase deficiency. Semin Hematol 1990; 27: 137–164.
Au WY, Ma SK, Lie AK, Liang R, Cheng T, Kwong YL . Glucose-6-phosphate dehydrogenase deficiency and hematopoietic stem cell transplantation. Bone Marrow Transplant 2002; 29: 399–402.
Au WY, So JC, Ma SK, Lie AK . Glucose-6-phosphate-dehydrogenase deficiency and haematopoietic stem cell transplantation in Chinese patients. Hong Kong Med J 2009; 15 (Suppl 3): 35–38.
Testa U, Meloni T, Lania A, Battistuzzi G, Cutillo S, Luzzatto L . Genetic heterogeneity of glucose 6-phosphate dehydrogenase deficiency in Sardinia. Hum Genet 1980; 56: 99–105.
Larghero J, Garcia J, Gluckman E . sources and procurement of stem cells. In: Haematology. ESo (ed) Hemopoietic Stem Cell Transplantation. The EBMT Handbook. 5th edn (Forum service editore: Genoa), 2008, pp 112–127.
Beutler E . Red Cell Metabolism. A Manual of Biochemical Methods, 2nd edn. Grune & Stratton: New York, 1975.
Ozer H, Armitage JO, Bennett CL, Crawford J, Demetri GD, Pizzo PA et al. 2000 update of recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. American Society of Clinical Oncology Growth Factors Expert Panel. J Clin Oncol 2000; 18: 3558–3585.
Baronciani D, Rambaldi A, Iori AP, Di Bartolomeo P, Pilo F, Pettinau M et al. Treosulfan/fludarabine as an allogeneic hematopoietic stem cell transplant conditioning regimen for high-risk patients. Am J Hematol 2008; 83: 717–720.
Gajewski JL, Johnson VV, Sandler SG, Sayegh A, Klumpp TR . A review of transfusion practice before, during, and after hematopoietic progenitor cell transplantation. Blood 2008; 112: 3036–3047.
Shalev O, Manny N, Sharon R . Posttransfusional hemolysis in recipients of glucose-6-phosphate dehydrogenase-deficient erythrocytes. Vox Sang 1993; 64: 94–98.
Huang CS, Sung YC, Huang MJ, Yang CS, Shei WS, Tang TK . Content of reduced glutathione and consequences in recipients of glucose-6-phosphate dehydrogenase deficient red blood cells. Am J Hematol 1998; 57: 187–192.
Acknowledgements
This clinical research was supported by AIL Associazione Italiana contro le Leucemie—Cagliari section. We thank Carlo Brugnara, MD for critical reading and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest
Rights and permissions
About this article
Cite this article
Pilo, F., Baronciani, D., Depau, C. et al. Safety of hematopoietic stem cell donation in glucose 6 phosphate dehydrogenase-deficient donors. Bone Marrow Transplant 48, 36–39 (2013). https://doi.org/10.1038/bmt.2012.112
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2012.112