Abstract
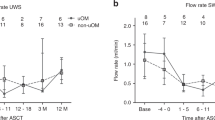
The incidence of long-term oral complications after hematopoietic SCT (HSCT) varies between 60 and 100%. The aim of this study was to compare the salivary secretion rate and the contribution of known risk factors for a low salivary secretion rate 1 year after HSCT in children conditioned with fractionated TBI (fTBI) and in children conditioned with single-dose TBI (sTBI). The study involved 44 patients, 27 conditioned with sTBI and 17 conditioned with fTBI. The unstimulated and stimulated salivary secretion rates (USSRs and SSSRs) were estimated before HSCT and at 1-year follow-up. Risk factors that may have influenced the salivary secretion rate were recorded. An SSSR of ⩽0.5 mL/min and a USSR of ⩽0.1 mL/min were chosen as cut-off points for salivary dysfunction. The median reduction in stimulated salivary flow 1 year after HSCT was 56% in the sTBI group and 12% in the fTBI group (P=0.003). The median reduction in unstimulated salivary flow 1 year after HSCT was 74% in the sTBI group and 33% in the fTBI group (P=0.003). In the multivariate model, a significant correlation between both sTBI (odds ratio (OR)=6.49, 95% confidence interval (CI)=1.40–30, P=0.014) and seropositivity of the recipient for 3–4 herpesviruses (OR=6.57, 95% CI=1.26–34, P=0.021) and a low stimulated salivary secretion rate (<0.5 mL/min) was found 1 year after HSCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Thomas ED, Fefer A, Buckner CD, Storb R . Current status of bone marrow transplantation for aplastic anemia and acute leukemia. Blood 1977; 49: 671–681.
Barrett AJ, Horowitz MM, Gale RP, Biggs JC, Camitta BM, Dicke KA et al. Marrow transplantation for acute lymphoblastic leukemia: factors affecting relapse and survival. Blood 1989; 74: 862–871.
Ringdén O, Le Blanc K . Allogeneic hematopoietic stem cell transplantation: state of the art and new perspectives. APMIS 2005; 113: 813–830.
Ringdén O, Hermans J, Labopin M, Apperley J, Gorin NC, Gratwohl A . The highest leukaemia-free survival after allogeneic bone marrow transplantation is seen in patients with grade I acute graft-versus-host disease. Acute and Chronic Leukaemia Working Parties of the European Group for Blood and Marrow Transplantation (EBMT). Leuk Lymphoma 1996; 24: 71–79.
Ringdén O, Deeg HJ (eds.) Clinical Spectrum of Graft-Versus-Host Disease, 2nd edn. Marcel Dekker Inc: New York, 1996.
Shimoni A, Hardan I, Shem-Tov N, Yeshurun M, Avigdor A, Ben-Bassat I . Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia 2006; 20: 322–328.
Alyea E, Kim H, Ho V, Cutler C, DeAngelo D, Stone R . Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant 2006; 12: 1047–1055.
Ringdén O . Allogeneic bone marrow transplantation for hematological malignancies-controversies and recent advances. Acta Oncol 1997; 36: 549–564.
Davies S, Ramsay N, Klein J, Weisdorf D, Bolwell B, Cahn J-Y et al. Comparison of preparative regimens in transplants for children with acute lymphoblastic leukemia. J Clin Oncol 2000; 18: 340–347.
Deeg HJ . Bone marrow transplantation: a review of delayed complications. Br J Haematol 1984; 57: 185–208.
Sanders JE . The impact of marrow transplant preparative regimens on subsequent growth and development. Semin Haematol 1991; 28: 244–249.
Ringdén O . Bone marrow transplantation using unrelated donors for haematological malignancies. Med Oncol 1997; 14: 11–22.
Clift R, Buckner D, Appelbaum F, Bearman S, Petersen F, Fisher L et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomised trial of two irradiation regimens. Blood 1990; 76: 1867–1871.
Epstein J, Haveman C, Huber M, Peterson D, Plemons J, Redding S et al. Oral health in cancer therapy. A guide for health care professionals. In: Rankin V, Gones D, Redding S (eds). Consensus Development Conference on Oral Health in Cancer Therapy by the Dental Oncology Education Programme, 3rd edn. Monographs 2008. Available from: http://www.nidcr.nih.gov.
Schubert M, Appelbaum F, Peterson D, Lloid M . Oral complications. In: Blume KG, Forman SJ (eds). Thomas’ Hematopoetic Cell Transplantation, 3rd edn. Blackwell Science Inc: Malden, MA, 2004, pp 911–928.
Dahllöf G, Heimdahl A, Bolme P, Lönnqvist B, Ringdén O . Oral condition in children treated with bone marrow transplantation. Bone Marrow Transplant 1988; 3: 43–51.
Dahllöf G, Krekmanova L, Kopp S, Borgström B, Forsberg CM, Ringdén O . Craniomandibular dysfunction in children treated with total-body irradiation and bone marrow transplantation. Acta Odontol Scand 1994; 52: 99–105.
Berkowitz RJ, Strandfjord S, Jones P, Hughes C, Barsetti J, Gordon EM et al. Stomatologic complications of bone marrow transplantation in a pediatric population. Pediatr Dent 1987; 9: 105–109.
Näsman M, Björk O, Söderhäll S, Ringdén O, Dahllöf G . Disturbances in the oral cavity in pediatrictic long-term survivors after different forms of antineoplastic therapy. Pediatr Dent 1994; 16: 217–223.
Dahllöf G, Bågesund M, Ringdén O . Impact of conditioning regimens on salivary function, caries associated microorganisms and dental caries in children after bone marrow transplantation. A 4-year longitudinal study. Bone Marrow Transplant 1997; 20: 479–483.
Dahllöf G, Bågesund M, Remberger M, Ringdén O . Risk factors for salivary dysfunction in children 1 year after bone marrow transplantation. Oral Oncol 1997; 33: 327–331.
Down JD, Tarbell NJ, Thames HD, Mauch PM . Syngeneic and allogeneic bone marrow engraftment after total body irradiation: dependence on dose, dose rate and fractionation. Blood 1991; 77: 661–669.
Adkins DR, Dipersio JF . Total body irradiation before an allogeneic stem cell transplantation: is there a magic dose? Curr Opin Hematol 2008; 15: 555–560.
Girinsky T, Benhamou E, Bourhis JH, Dhermain F, Guilliot-Valls D, Ganansia V et al. Prospective randomized comparison of single-dose versus hyperfractionated total body irradiation in patients with hematologic malignancies. J Clin Oncol 2000; 18: 981–986.
Aristei C, Allesandro M, Santucci A, Aversa F, Tabillo A, Carotti A et al. Cataracts in patients receiving stem cell transplantation after conditioning with total body irradiation. Bone Marrow Transplant 2002; 29: 503–507.
Cosset JM, Girinsky T, Malaise E, Chaillet MP, Dutreix J . Clinical basis for TBI fractionation. Radiother Oncol Suppl 11990; 18 (Suppl 1): 60–67.
Oya N, Sasai K, Tachiiri S, Sakamoto T, Nagata Y, Okada T et al. Influence of radiation dose rate and lung dose on interstitial pneumonitis after fractionated total body irradiation: acute parotitis may predict interstitial pneumonitis. Int J Hematol 2006; 83: 86–91.
Jensen SB, Pedersen ML, Vissink A, Andersen E, Brown CG, Davies AN et al. A systematic review of salivary hypofunction and xerestomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support Care Cancer 2010; 18: 1061–1079.
Jen YM, Lin YC, Wang YB, Wu DM . Dramatic prolonged decrease of whole salivary secretion in nasopharyngeal carcinoma patients treated with radiotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006; 101: 322–327.
Buchali A, Feyer P, Groll J, Massenkeil G, Arnold R, Budach V . Immediate toxicity during fractionated total body irradiation as conditioning for bone marrow transplantation. Radiother Oncol 2000; 54: 157–162.
Bågesund M, Winiarski J, Dahllöf G . Subjective xerostomia in long-term surviving children and adolescents after pediatric bone marrow transplantation. Transplantation 2000; 69: 822–826.
Taylor SE, Miller EG . Preemptive pharmacological intervention in radiation-induced salivary dysfunction. Proc Soc Exp Biol Med 1999; 221: 14–26.
Kashima HK, Kirkham WR, Andrews JR . Postirradiation sialodenitis. Am J Roentgenol Radium Ther Nucl Med 1965; 94: 271–291.
Yagil C, Michaeli Y, Zajicek G . Compensatory proliferative response of the rat submandibular salivary gland to unilateral extirpation. Virchows Arch B Cell Pathol Incl Mol Pathol 1985; 49: 83–91.
Stephens CL, Schultheiss TE, Price RE, Ang KK, Peters LJ . Radiation apoptosis of serous acinar cells of salivary and lacrimal glands. Cancer 1991; 67: 1539–1543.
Wollf A, Limor ZP, Kaplan I . Major salivary gland output differs between users and non-users of specific medication categories. Gerodontology 2008; 25: 210–216.
Shillitoe EJ, Daniels TE, Whitcher JP, Strand CV, Talal N, Greenspan JS . Antibody to cytomegalovirus in patients with Sjögren's syndrome. Arthritis Rheum 1982; 25: 260–265.
Fox RI, Luppi M, Pisa P, Kang HI . Potential role of Epstein-Barr virus in Sjögren's syndrome and rheumatoid arthritis. J Rheumatol Suppl 1992; 32: 18–24.
Yamaoka K, Miyasaka N, Yamamoto K . Possible involvement of Epstein-Barr virus polyclonal B cell activation in Sjögrens syndrome. Arthritis Rheum 1988; 31: 1014–1021.
Fox JD, Briggs M, Ward P, Tedder R . Human herpesvirus 6 in salivary glands. Lancet 1990; 336: 590–593.
Imanguli M, Atkinson J, Mitchell S, Avila D, Bishop R, Cowen E et al. Salivary gland involvement in chronic graft-versus-host disease: prevalence, clinical significance, and recommendations for evaluation. Biol Blood Marrow Transpl 2010; 16: 1362–1369.
Heimdahl A, Johnson G, Danielsson KH, Lönnqvist B, Ringdén O . Oral condition of patients with leukaemia and aplastic anemia. Oral Surg Oral Med Oral Pathol 1985; 60: 498–504.
Ringdén O, Deeg HJ . Clinical spectrum of graft-versus-host disease. In: Ferrara JLM, Deeg HJ, Burakoff S (eds). Graft vs Host Disease, 2nd edn. Marcel Dekker, Inc: New York, 1996, pp 525–559.
Acknowledgements
This study was supported by grants from the Swedish Dental Society, Swedish Medical Society, Swedish Childhood Cancer Foundation and Karolinska Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Legert, K., Remberger, M., Ringdèn, O. et al. Salivary secretion in children after fractionated or single-dose TBI. Bone Marrow Transplant 47, 404–410 (2012). https://doi.org/10.1038/bmt.2011.96
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2011.96
Keywords
This article is cited by
-
Oral and dental late effects in survivors of childhood cancer: a Children’s Oncology Group report
Supportive Care in Cancer (2014)