Abstract
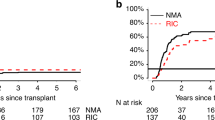
Although reduced-intensity conditioning (RIC) and non-myeloablative (NMA)-conditioning regimens have been used for over a decade, their relative efficacy vs myeloablative (MA) approaches to allogeneic hematopoietic cell transplantation in patients with AML and myelodysplasia (MDS) is unknown. We compared disease status, donor, graft and recipient characteristics with outcomes of 3731 MA with 1448 RIC/NMA procedures performed at 217 centers between 1997 and 2004. The 5-year univariate probabilities and multivariate relative risk outcomes of relapse, TRM, disease-free survival (DFS) and OS are reported. Adjusted OS at 5 years was 34, 33 and 26% for MA, RIC and NMA transplants, respectively. NMA conditioning resulted in inferior DFS and OS, but there was no difference in DFS and OS between RIC and MA regimens. Late TRM negates early decreases in toxicity with RIC and NMA regimens. Our data suggest that higher regimen intensity may contribute to optimal survival in patients with AML/MDS, suggesting roles for both regimen intensity and graft vs leukemia in these diseases. Prospective studies comparing regimens are needed to confirm this finding and determine the optimal approach to patients who are eligible for either MA or RIC/NMA conditioning.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Vicente D, Lamparelli T, Gualandi F, Occhini D, Raiola AM, Ibatici A et al. Improved outcome in young adults with de novo acute myeloid leukemia in first remission, undergoing an allogeneic bone marrow transplant. Bone Marrow Transplant 2007; 40: 349–354.
Blaise D, Maraninchi D, Archimbaud E, Reiffers J, Devergie A, Jouet JP et al. Allogeneic bone marrow transplantation for acute myeloid leukemia in first remission: a randomized trial of a busulfan-cytoxan versus cytoxan-total body irradiation as preparative regimen: a report from the Group d’Etudes de la Greffe de Moelle Osseuse. Blood 1992; 79: 2578–2582.
Chang C, Storer BE, Scott BL, Bryant EM, Shulman HM, Flowers ME et al. Hematopoietic cell transplantation in patients with myelodysplastic syndrome or acute myeloid leukemia arising from myelodysplastic syndrome: similar outcomes in patients with de novo disease and disease following prior therapy or antecedent hematologic disorders. Blood 2007; 110: 1379–1387.
Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 1998; 91: 756–763.
McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 2001; 97: 3390–3400.
Giralt S, Estey E, Albitar M, van Besien K, Rondon G, Anderlini P et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood 1997; 89: 4531–4536.
Khouri IF, Keating M, Korbling M, Przepiorka D, Anderlini P, O′Brien S et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol 1998; 16: 2817–2824.
Hegenbart U, Niederwieser D, Sandmaier BM, Maris MB, Shizuru JA, Greinix H et al. Treatment for acute myelogenous leukemia by low-dose, total-body, irradiation-based conditioning and hematopoietic cell transplantation from related and unrelated donors. J Clin Oncol 2006; 24: 444–453.
Gratwohl A, Baldomero H, Frauendorfer K, Urbano-Ispizua A . EBMT activity survey 2004 and changes in disease indication over the past 15 years. Bone Marrow Transplant 2006; 37: 1069–1085.
Bertz H, Potthoff K, Finke J . Allogeneic stem-cell transplantation from related and unrelated donors in older patients with myeloid leukemia. J Clin Oncol 2003; 21: 1480–1484.
Martino R, Caballero MD, Perez-Simon JA, Canals C, Solano C, Urbano-Ispizua A et al. Evidence for a graft-versus-leukemia effect after allogeneic peripheral blood stem cell transplantation with reduced-intensity conditioning in acute myelogenous leukemia and myelodysplastic syndromes. Blood 2002; 100: 2243–2245.
Tauro S, Craddock C, Peggs K, Begum G, Mahendra P, Cook G et al. Allogeneic stem-cell transplantation using a reduced-intensity conditioning regimen has the capacity to produce durable remissions and long-term disease-free survival in patients with high-risk acute myeloid leukemia and myelodysplasia. J Clin Oncol 2005; 23: 9387–9393.
Mohty M, Bay JO, Faucher C, Choufi B, Bilger K, Tournilhac O et al. Graft-versus-host disease following allogeneic transplantation from HLA-identical sibling with antithymocyte globulin-based reduced-intensity preparative regimen. Blood 2003; 102: 470–476.
Niederwieser D, Maris M, Shizuru JA, Petersdorf E, Hegenbart U, Sandmaier BM et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood 2003; 101: 1620–1629.
Flynn CM, Hirsch B, Defor T, Barker JN, Miller JS, Wagner JE et al. Reduced intensity compared with high dose conditioning for allotransplantation in acute myeloid leukemia and myelodysplastic syndrome: a comparative clinical analysis. Am J Hematol 2007; 82: 867–872.
Scott BL, Sandmaier BM, Storer B, Maris MB, Sorror ML, Maloney DG et al. Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: a retrospective analysis. Leukemia 2006; 20: 128–135.
Massenkeil G, Nagy M, Neuburger S, Tamm I, Lutz C, le Coutre P et al. Survival after reduced-intensity conditioning is not inferior to standard high-dose conditioning before allogeneic haematopoietic cell transplantation in acute leukaemias. Bone Marrow Transplant 2005; 36: 683–689.
Alyea EP, Kim HT, Ho V, Cutler C, DeAngelo DJ, Stone R et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant 2006; 12: 1047–1055.
Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2009; 15: 367–369.
Giralt S, Logan B, Rizzo D, Zhang MJ, Ballen K, Emmanouilides C et al. Reduced-intensity conditioning for unrelated donor progenitor cell transplantation: long-term follow-up of the first 285 reported to the national marrow donor program. Biol Blood Marrow Transplant 2007; 13: 844–852.
Gooley TA, Leisenring W, Crowley J, Storer BE . Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 695–706.
Klein J, Moeschberger M . Survival Analysis: Techniques of Censored and Truncated Data. 2nd edn Springer-Verlag: New York, NY, 2003.
Aoudjhane M, Labopin M, Gorin NC, Shimoni A, Ruutu T, Kolb HJ et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Leukemia 2005; 19: 2304–2312.
Ringden O, Labopin M, Ehninger G, Niederwieser D, Olsson R, Basara N et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol 2009; 27: 4570–4577.
Report from the International Bone Marrow Transplant Registry. Advisory committee of the international bone marrow transplant registry. Bone Marrow Transplant 1989; 4: 221–228.
Martino R, Iacobelli S, Brand R, Jansen T, van Biezen A, Finke J et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood 2006; 108: 836–846.
Acknowledgements
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases; a grant/cooperative agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen Inc.; anonymous donation to the Medical College of Wisconsin; Astellas Pharma US Inc.; Baxter International Inc.; Bayer HealthCare Pharmaceuticals; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical Inc.; Biovitrum AB; Blood Center of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Canadian Blood and Marrow Transplant Group; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Centers for Disease Control and Prevention; Children's Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex Inc.; CytoTherm; DOR BioPharma Inc.; Dynal Biotech, an Invitrogen Company; Eisai Inc.; Enzon Pharmaceuticals Inc.; European Group for Blood and Marrow Transplantation; Gamida Cell Ltd; GE Healthcare; Genentech Inc.; Genzyme Corporation; Histogenetics Inc.; HKS Medical Information Systems; Hospira Inc.; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co. Ltd; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; MGI Pharma Inc.; Michigan Community Blood Centers; Millennium Pharmaceuticals Inc.; Miller Pharmacal Group; Milliman USA Inc.; Miltenyi Biotec Inc.; NMDP; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics Inc.; Otsuka America Pharmaceutical Inc.; Pall Life Sciences; Pfizer Inc.; Saladax Biomedical Inc.; Schering Corporation; Society for Healthcare Epidemiology of America; StemCyte Inc.; StemSoft Software Inc.; Sysmex America Inc.; Teva Pharmaceutical Industries; Therakos Inc.; Thermogenesis Corporation; Vidacare Corporation; Vion Pharmaceuticals Inc.; ViraCor Laboratories; ViroPharma Inc.; and Wellpoint Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense or any other agency of the US Government. We thank Karen Ballen, Willem Bujan, Steven Goldstein, Roger Herzig, Osman Ilhan, Luis Isola, Jane Liesveld, Gustavo Milone, David Rizzieri, James Russell, Mitchell Sabloff, Stella Santarone, Gary Schiller, Robert Soiffer, Edmund Waller, Roy Weiner and Peter Wiernik for helpful comments and insights.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Luger, S., Ringdén, O., Zhang, MJ. et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant 47, 203–211 (2012). https://doi.org/10.1038/bmt.2011.69
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2011.69
Keywords
This article is cited by
-
CLAG combined with total body irradiation as intensive conditioning chemotherapy prior to allogeneic hematopoietic stem cell transplantation in patients with refractory or relapsed acute myeloid leukemia
Annals of Hematology (2024)
-
Clofarabine and total body irradiation (TBI) as conditioning regimen for allogeneic stem cell transplantation in high-risk acute leukemia patients: a two-center retrospective cohort study
Bone Marrow Transplantation (2023)
-
Comparison of reduced intensity and nonmyeloablative conditioning for adults with acute myeloid leukemia undergoing allogeneic hematopoietic cell transplantation in first or second remission
Bone Marrow Transplantation (2023)
-
Age and allogeneic hematopoietic cell transplantation outcomes in acute myeloid leukemia
International Journal of Hematology (2023)
-
Impact of induction chemotherapy with intermediate-dosed cytarabine and subsequent allogeneic stem cell transplantation on the outcome of high-risk acute myeloid leukemia
Journal of Cancer Research and Clinical Oncology (2022)