Abstract
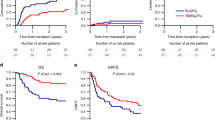
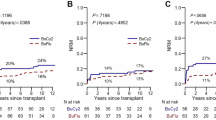
We conducted a retrospective study to evaluate the outcome of 94 consecutive patients with high-risk hematological malignancies who received allo-PBSCT, following idarubicin (IDA)-intensified BUCY2 (IDA-BUCY2) myeloablative conditioning regimens (n=53) and BUCY2 conditioning regimens (n=41). IDA 15 mg/m2 once daily was administered by continuous infusion on days −11 to −9, followed by BU, 3.2 mg/kg in divided doses daily, on days −6 to −4, and i.v. injection of CY, 1.8 g/m2 once daily on days −3 to −2 in the IDA-BUCY2 group. The relapse rate in patients in the IDA-BUCY2 and BUCY2-conditioning regimens group was 18.9 and 39%, respectively (P=0.030). There was no significant difference in terms of TRM. The cumulative probabilities of OS and disease-free survival at 2 years for patients conditioned with the IDA-BUCY2 and BUCY2 regimens were 65.3% vs 46.8% (P=0.038), and 63.5% vs 43.4% (P=0.025), respectively. Multivariate analysis showed that IDA-BUCY2 regimens and limited chronic GVHD were the only two factors resulting in improved survival and reduced relapse rate. This retrospective study suggests that IDA-intensified BUCY2 may be substituted for BUCY2 as conditioning regimen for patients with high-risk hematological malignancies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Zittoun RA, Mandelli F, Willemze R, De Witte T, Labar B, Resegotti L et al. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia: European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Malignedell’Adulto (GIMEMA) Leukemia Cooperative Groups. N Engl J Med 1995; 332: 217–223.
Appelbaum FR . Haematopoietic cell transplantation as immunotherapy. Nature 2001; 411: 385–389.
Lee KH, Lee JH, Choi SJ, Lee JH, Kim S, Seol M et al. Bone marrow vs extramedullary relapse of acute leukemia after allogeneic hematopoietic cell transplantation: risk factors and clinical course. Bone Marrow Transplant 2003; 32: 835–842.
Aoudjhane M, Labopin M, Gorin NC, Shimoni A, Ruutu T, Kolb HJ et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Leukemia 2005; 19: 2304–2312.
Valcárcel D, Martino R, Caballero D, Martin J, Ferra C, Nieto JB et al. Sustained remissions of high-risk acute myeloid leukemia and myelodysplastic syndrome after reduced-intensity conditioning allogeneic hematopoietic transplantation: chronic graft-versus-host disease is the strongest factor improving survival. J Clin Oncol 2008; 26: 577–584.
Beelen DW, Trenschel R, Casper J, Freund M, Hilger RA, Scheulen ME et al. Dose-escalated treosulphan in combination with cyclophosphamide as a new preparative regimen for allogeneic haematopoietic stem cell transplantation in patients with an increased risk for regimen-related complications. Bone Marrow Transplant 2005; 35: 233–241.
Aschan J . Risk assessment in haematopoietic stem cell transplantation: conditioning. Best Pract Res Clin Haematol 2007; 20: 295–310.
Mengarelli A, Iori AP, Guglielmi C, Romano A, Cerretti R, Torromeo C et al. Standard versus alternative myeloablative conditioning regimens in allogeneic hematopoietic stem cell transplantation for high-risk acute leukemia. Haematologica 2002; 87: 52–58.
Toubai T, Tanaka J, Mori A, Hashino S, Kobayashi S, Ota S et al. Efficacy of etoposide, cyclophosphamide, and total body irradiation in allogeneic bone marrow transplantation for adult patients with hematological malignancies. Clin Transplant 2004; 18: 552–557.
Mengarelli A, Iori AP, Guglielmi C, Perrone MP, Gozzer M et al. Idarubicin intensified BUCY2 regimen in allogeneic unmanipulated transplant for high-risk hematological malignancies. Leukemia 2000; 14: 2052–2058.
Shigematsu A, Kondo T, Yamamoto S, Sugita J, Onozawa M, Kahata K et al. Excellent outcome of allogeneic hematopoietic stem cell transplantation using a conditioning regimen with medium-dose VP-16, cyclophosphamide and total-body irradiation for adult patients with acute lymphoblastic leukemia. Biol Blood Marrow Transplant 2008; 14: 568–575.
Zohren F, Czibere A, Bruns I, Fenk R, Schroeder T, Graf T et al. Fludarabine, amsacrine, high-dose cytarabine and 12 Gy total body irradiation followed by allogeneic hematopoietic stem cell transplantation is effective in patients with relapsed or high-risk acute lymphoblastic leukemia. Bone Marrow Transplant 2009; 44: 785–792.
Mandelli F, Vignetti M, Suciu S, Stasi R, Petti MC, Meloni G et al. Daunorubicin versus mitoxantrone versus idarubicin as induction and consolidation chemotherapy for adults with acute myeloid leukemia: the EORTC and GIMEMA Groups Study AML-10. J Clin Oncol 2009; 27: 5397–5403.
Reid JM, Pendergrass TW, Krailo MD, Hammond GD, Ames MM . Plasma pharmacokinetics and cerebrospinal fluid concentrations of idarubicin and idarubicinol in pediatric leukemia patients: a Children's Cancer Study Group report. Cancer Res 1990; 50: 6525–6528.
Case DC, Gerber MC, Gams RA, Crawford J, Votaw ML, Higano CS et al. Phase II study of intravenous idarubicin in unfavourable non-Hodgkin's lymphoma. Leuk Lymphoma 1993; 10: 73–79.
Ferrara F, Palmieri S, Annunziata M, Viola A, Pocali B, Califano C et al. Continuous infusion idarubicin and oral busulfan as conditioning for patients with acute myeloid leukemia aged over 60 years undergoing autologous stem cell transplantation. Bone Marrow Transplant 2004; 34: 573–576.
Ferrara F, Palmieri S, De Simone M, Sagristani M, Viola A, Pocali B et al. High-dose idarubicin and busulphan as conditioning to autologous stem cell transplantation in adult patients with acute myeloid leukaemia. Br J Haematol 2005; 128: 234–241.
Ferrara F, Palmieri S, Pedata M, Viola A, Izzo T, Criscuolo C et al. Autologous stem cell transplantation for elderly patients with acute myeloid leukaemia conditioned with continuous infusion idarubicin and busulphan. Hematol Oncol 2009; 27: 40–45.
Ferrara F, Mele G, Palmieri S, Pedata M, Copia C, Riccardi C et al. Continuous infusion idarubicin and intravenous busulphan as conditioning regimen to autologous stem cell transplantation for patients with acute myeloid leukaemia. Hematol Oncol 2009; 27: 198–202.
Hunault M, Harousseau JL, Delain M, Truchan-Graczyk M, Cahn JY, Witz F et al. Better outcome of adult acute lymphoblastic leukemia after early genoidentical allogeneic bone marrow transplantation (BMT) than after late high-dose therapy and autologous BMT : a GOEL-AMS trial. Blood 2004; 104: 3028–3037.
Kern W, Haferlach T, Schoch C, Loffler H, Gassmann W, Heinecke A et al. Early blast clearance by remission induction chemotherapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia:data from the German AML cooperative group (AMLCG) 1992 trial. Blood 2003; 101: 64–70.
Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G et al. The importance of diagnostic cytogenetics on outcome in AML:Analysis of 1,612 patients entered into the MRCAML10 trial—The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood 1998; 92: 2322–2333.
Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a South west Oncology Group/Eastern Cooperative Oncology Group Study. Blood 2000; 96: 4075–4083.
Schoch C, Haferlach T, Haase D, Fonatsch C, Löffler H, Schlegelberger B et al. Patients with denovo acute myeloid leukaemia and complex karyotype aberrations show a poor prognosis despite intensive treatment: a study of 90 patients. Br J Haematol 2001; 112: 118–126.
Appelbaum FR . Who should be transplanted for AML. Leukemia 2001; 15: 680–682.
Schoch C, Kern W, Schnittger S, Hiddemann W, Haferlach T . Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): An analysis of 93 patients with t-AML in comparison to 1092 patients with de novo AML. Leukemia 2004; 18: 120–125.
Sanz GF, Sanz MA, Vallespi T, Canizo MC, Torrabadella M, Garcia S et al. Two regression models and a scoring system for predicting survival and planning treatment in myelodysplastic syndrome: a multivariate analysis of prognostic factors in 370 patients. Blood 1989; 74: 395–408.
Greenberg P, Cox C, Le Beau MM, Fenaux P, Morel P, Sanz G et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997; 89: 2079–2088.
Thiede C, Florek M, Bornhäuser M, Ritter M, Mohr B, Brendel C et al. Rapid quantication of mixed chimerism using multiplex amplification of short tandem repeat markers and uorescence detection. Bone Marrow Transplant 1999; 23: 1055–1060.
Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/hapl oidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood 2006; 107: 3065–3073.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Sullivan KM . Graft-versus-host disease. In: Thomas ED, Blume KG, Forman SJ (eds). Hematopoietic Cell Transplantation. Blackwell Scientific: London, Oxford, 1999, pp 515–536.
Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol 1988; 6: 1562–1568.
Kaplan EL, Meier P . Non parametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Cox DR . Regression models and life-tables. JR Stat Soc 1972; 34: 187–189.
Ferrara F, Palmieri S, De Simone M, Sagristani M, Viola A, Pocali B et al. High-dose idarubicin and busulphan as conditioning to autologous stem cell transplantation in adult patients with acute myeloid leukaemia. Br J Haematol 2005; 128: 234–241.
Ferrara F, Annunziata M, Schiavone EM, Copia C, De Simone M, Pollio F et al. High-dose idarubicin and busulphan as conditioning for autologous stem cell transplantation in acute myeloid leukemia: a feasibility study. Hematol J 2001; 2: 214–219.
Jerjis S, Roovers E, Muus P, Schaap N, De Witte T . Idarubicin to intensify the conditioning regimens of autologous bone marrow transplantation for patients with acute myeloid leukemia in first complete remission. Bone Marrow Transplant 1998; 22: 13–19.
Brown RA, Wolff SN, Fay JW, Pineiro L, Collins Jr RH, Lynch JP et al. High-dose etoposide, cyclophosphamide and total body irradiation with allogeneic bone marrow transplantation for resistant acute myeloid leukemia: a study by the North American Marrow Transplant Group. Leuk Lymphoma 1996; 22: 271–277.
Hamaki T, Kami M, Kanda Y, Yuji K, Inamoto Y, Kishi Y et al. Reduced-intensity stem-cell transplantation for adult acute lymphoblastic leukemia: a retrospective study of 33 patients. Bone Marrow Transplant 2005; 35: 549–556.
Mohty M, Labopin M, Volin L, Gratwohl A, Socié G, Esteve J et al. Reduced intensity conditioning allogeneic stem cell transplantation for adult patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Haematologica 2008; 93: 303–306.
Stein AS, Palmer JM, O’Donnell MR, Kogut NM, Spielberger RT, Slovak ML et al. Reduced-intensity conditioning followed by peripheral blood stem cell transplantation for adult patients with high-risk acute lymphoblastic leukemia. Biol Blood Marrow Transplant 2009; 15: 1407–1414.
Aschan J . Allogeneic haematopoietic stem cell transplantation: current status and future outlook. Br Med Bull 2006; 77–78: 23–36.
Lee S, Cho BS, Kim SY, Choi SM, Lee DG, Eom KS et al. Allogeneic stem cell transplantation in first complete remission enhances graft-versus-leukemia effect in adults with acute lymphoblastic leukemia: antileukemic activity of chronic graft-versus-host disease. Biol Blood Marrow Transplant 2007; 13: 1083–1094.
Lake RA, Robinson BW . Immunotherapy and chemotherapy—a practical partnership. Nat Rev Cancer 2005; 5: 397–405.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hong, M., Wu, Q., Hu, C. et al. Idarubicin-intensified BUCY2 regimens may lower relapse rate and improve survival in patients undergoing allo-SCT for high-risk hematological malignancies: a retrospective analysis. Bone Marrow Transplant 47, 196–202 (2012). https://doi.org/10.1038/bmt.2011.66
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2011.66
Keywords
This article is cited by
-
Minimal residual disease monitoring and preemptive immunotherapies for frequent 11q23 rearranged acute leukemia after allogeneic hematopoietic stem cell transplantation
Annals of Hematology (2021)
-
Unmanipulated haplo-identical donor transplantation compared with identical sibling donor had better anti-leukemia effect for refractory/relapsed acute myeloid leukemia not in remission status
Annals of Hematology (2020)
-
Idarubicin-intensified haploidentical HSCT with GvHD prophylaxis of ATG and basiliximab provides comparable results to sibling donors in high-risk acute leukemia
Bone Marrow Transplantation (2017)