Abstract
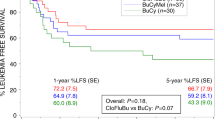
Relapse is the major cause of mortality in patients with acute myeloid leukemia (AML) after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Effective preventive intervention in high-risk AML may be crucial. In this study, we investigated the clinical efficacy and safety of low dose decitabine (DAC) as part of a modified Busulfan-Cyclophosphamide (Bu-Cy) regimen for high-risk AML patients undergoing allo-HSCT to reduce relapse rate. Fifty-nine patients received DAC (20 mg/m2/d, i.v.) for 5 days, followed by modified Bu-Cy (DAC group). A matched-pair control (CON) group of 177 patients (matched 1:3) received modified Bu-Cy only. The differences were more substantial among patients with active disease: 2-year OS, 80.7% (DAC) versus 43.5% (CON), P = 0.011 and 2-year LFS, 64.9% (DAC) versus 39.2% (CON), P = 0.024. Median time to relapse was 8 months (DAC) versus 5 months (CON) for the entire groups and 6.5 months (DAC) versus 3.5 months (CON) for patients with active disease. In summary, our data indicated that the conditioning regimen containing low dose DAC may confer a survival advantage in high-risk AML patients with active disease undergoing allo-HSCT, and a prospective randomized trial is warranted to confirm these observations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127:62–70.
Barrett AJ, Battiwalla M. Relapse after allogeneic stem cell transplantation. Expert Rev Hematol. 2010;3:429–41.
Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373:1136–52.
Shimoni A, Hardan I, Shem-Tov N, Yeshurun M, Yerushalmi R, Avigdor A, et al. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia. 2006;20:322–8.
Bacigalupo A, Sormani MP, Lamparelli T, Gualandi F, Occhini D, Bregante S, et al. Reducing transplant-related mortality after allogeneic hematopoietic stem cell transplantation. Haematologica. 2004;89:1238–47.
Cornelissen JJ, van Putten WL, Verdonck LF, Theobald M, Jacky E, Daenen SM, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood. 2007;109:3658–66.
Momparler RL. Molecular, cellular and animal pharmacology of 5-aza-2’-deoxycytidine. PharmacolTher. 1985;30:287–99.
Valdez BC, Li Y, Murray D, Corn P, Champlin RE, Andersson BS. 5-Aza-2’-deoxycytidine sensitizes busulfan-resistant myeloid leukemia cells by regulating expression of genes involved in cell cycle checkpoint and apoptosis. Leuk Res. 2010;34:364–72.
Choi J, Ritchey J, Prior JL, Holt M, Shannon WD, Deych E, et al. In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood. 2010;116:129–39.
Valdez BC, Tang X, Li Y, Murray D, Liu Y, Popat U, et al. Epigenetic modification enhances the cytotoxicity of busulfan and 4-hydroperoxycyclophosphamide in AML cells. Exp Hematol. 2018;67:49–59.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Lee SJ, Vogelsang G, Flowers MED. Chronic graft-versus-host disease. Biol Blood MarrowTransplant. 2003;9:215–33.
Kaplan EL, Meier P. Nonparametric estimator from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Vijayaraghavalu S, Labhasetwar V. Efficacy of decitabine-loaded nanogels in overcoming cancer drug resistance is mediated via sustained DNA methyltransferase 1 (DNMT1) depletion. Cancer Lett. 2013;331:122–9.
Li K, Hu C, Mei C, Ren Z, Vera JC, Zhuang Z, et al. Sequential combination of decitabine and idarubicin synergistically enhances anti-leukemia effect followed by demethylating Wnt pathway inhibitor promoters and downregulating Wnt pathway nuclear target. J Transl Med. 2014;12:167–80.
Sigalotti L, Altomonte M, Colizzi F, Degan M, Rupolo M, Zagonel V, et al. 5-Aza-2’-deoxycytidine (decitabine) treatment of hematopoietic malignancies: a multimechanism therapeutic approach? Blood. 2003;101:4644–6.
Almstedt M, Pfeifer D, Lubbert M. Cancer testis antigens residing on the X-chromosome are preferentially derepressed in myeloid leukemic cells by the DNA demethylating agent 5-aza-2 0 -deoxycytidine (DAC). Blood. 2008;112:abstr 2247.
Klar AS, Gopinadh J, Kleber S, Wadle A, Renner C. Treatment with 5-aza-2’-deoxycytidine induces expression of NY-ESO-1 and facilitates cytotoxic T lymphocyte-mediated tumor cell killing. Plos ONE. 2015;10:e139221–139235.
Jing Y, Shen X, Mei Q, Han W. Spotlight on decitabine for myelodysplastic syndromes in Chinese patients. Onco Targets Ther. 2015;8:2783–90.
Cany J, Roeven MWH, Hoogstad-van Evert JS, Hobo W, Maas F, Franco Fernandez R, et al. Decitabine enhances targeting of AML cells by CD34(+) progenitor-derived NK cells in NOD/SCID/IL2Rg(null) mice. Blood. 2018;131:202–14.
Wang LX, Mei ZY, Zhou JH, Yao YS, Li YH, Xu YH, et al. Low dose decitabine treatment induces CD80 expression in cancer cells and stimulates tumor specific cytotoxic T lymphocyte responses. Plos ONE. 2013;8:e62924–e62935.
Ma Y, Qu C, Dai H, Yin J, Li Z, Chen J, et al. Maintenance therapy with decitabine after allogeneic hematopoietic stem cell transplantation to prevent relapse of high-risk acute myeloid leukemia. Bone Marrow Transplant. 2020;55:1206–8.
Ravandi F, Issa JP, Garcia-Manero G, O’Brien S, Pierce S, Shan J, et al. Superior outcome with hypomethylating therapy in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome and chromosome 5 and 7 abnormalities. Cancer 2009;115:5746–51.
Lübbert M, Rüter BH, Claus R, Schmoor C, Schmid M, Germing U, et al. A multicenter phase II trial of decitabine as first-line treatment for older patients with acute myeloid leukemia judged unfit for induction chemotherapy. Haematologica. 2012;97:393–401.
Song G, Valdez BC, Li Y, Liu Y, Champlin RE, Andersson BS. Synergistic toxicity of sorafenib with busulfan and nucleoside analogs in human FMS-like tyrosine kinase 3 internal tandem duplications-positive acute myeloid leukemia cells. Biol Blood Marrow Transplant. 2014;20:1687–95.
Im AP, Sehgal AR, Carroll MP, Smith BD, Tefferi A, Johnson DE, et al. DNMT3A and IDH mutations in acute myeloid leukemia and other myeloid malignancies: associations with prognosis and potential treatment strategies. Leukemia. 2014;28:1774–83.
Bejar R, Lord A, Stevenson K, Bar-Natan M, Pérez-Ladaga A, Zaneveld J, et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood. 2014;124:2705–12.
Giralt S, Davis M, O’Brien S, van Besien K, Champlin R, de Vos D, et al. Studies of decitabine with allogeneic progenitor cell transplantation. Leukemia. 1997;11:S32–S34.
de Lima M, Ravandi F, Shahjahan M, Andersson B, Couriel D, Donato M, et al. Long-term follow-up of a phase I study of high-dose decitabine, busulfan, and cyclophosphamide plus allogeneic transplantation for the treatment of patients with leukemias. Cancer 2003;97:1242–7.
Acknowledgements
This work was supported by research grants from National Natural Science Foundation of China (81873443, 82070162, 81900175, 81400155, 81700139), Jiangsu Provincial Medical Talent (ZDRCA2016045), Major Natural Science Research Projects in institutions of higher education of Jiangsu Province (19KJA210002), The Key Science Research Project of Jiangsu Commission of Health (K2019022), Translational Research Grant of NCRCH (2020ZKZC04) and Natural Science Foundation of Jiangsu Province (BK20190181, BK20201169), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tang, X., Valdez, B.C., Ma, Y. et al. Low-dose decitabine as part of a modified Bu-Cy conditioning regimen improves survival in AML patients with active disease undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 56, 1674–1682 (2021). https://doi.org/10.1038/s41409-021-01238-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-021-01238-5
This article is cited by
-
Decitabine-containing conditioning improved outcomes for children with higher-risk myelodysplastic syndrome undergoing allogeneic hematopoietic stem cell transplantation
Annals of Hematology (2024)
-
Decitabine combined with minimally myelosuppressive therapy for induction of remission in pediatric high-risk acute myeloid leukemia with chromosome 5q deletion: a report of three cases
International Journal of Hematology (2022)