Abstract
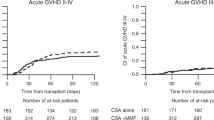
We investigated sirolimus and mycophenolate mofetil (MMF) as GVHD prophylaxis in patients with advanced hematological malignancies receiving myeloablative hematopoietic cell transplantation (HCT) from HLA-identical sibling donors. On the basis of pre-study stopping rules, the trial was closed to accrual after enrollment of 11 adult patients. In all, 7 of the 11 patients received BU-containing preparative regimens. Sirolimus was discontinued in three patients because of the toxicity-related events of severe sinusoidal obstructive syndrome, portal vein thrombosis, altered mental status and in one patient because of the risk of poor wound healing. In all, 6 of the 11 patients developed grade II–IV acute GVHD (AGVHD) a median of 15.5 days post HCT. Two of three patients with grade IV AGVHD had sirolimus discontinued by 9 days post HCT. All patients responded to AGVHD therapy without GVHD-related deaths. There were two non-relapse- and two relapse-related deaths. At a median follow-up of 38 months (2–47 months), 7 of 11 patients were alive without disease. MMF and sirolimus GVHD prophylaxis did not reduce the risk of AGVHD, however, there were no GVHD-related deaths. The severe toxicities in the patients receiving the BU-containing preparative regimens limited the continued use of sirolimus and MMF for the prevention of AGVHD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kahan BD, Camardo JS . Rapamycin: clinical results and future opportunities. Transplantation 2001; 72: 1181–1193.
Benito AI, Furlong T, Martin PJ, Anasetti C, Appelbaum FR, Doney K et al. Sirolimus (rapamycin) for the treatment of steroid-refractory acute graft-versus-host disease. Transplantation 2001; 72: 1924–1929.
Cutler C, Li S, Ho VT, Koreth J, Alyea E, Soiffer RJ et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood 2007; 109: 3108–3114.
Chao NJ . Pharmacology and use of immunosuppressive agents after hematopoietic cell transplantation. In: Thomas E, Blume K, Forman S (eds) Hematopoietic Cell Transplantation, 2nd edn. Blackwell: Malden, MA, 1999 pp 176–185.
Cutler C, Li S, Kim HT, Laglenne P, Szeto KC, Hoffmeister L et al. Mucositis after allogeneic hematopoietic stem cell transplantation: a cohort study of methotrexate- and non-methotrexate-containing graft-versus-host disease prophylaxis regimens. Biol Blood Marrow Transplant 2005; 11: 383–388.
Marty FM, Bryar J, Browne SK, Schwarzberg T, Ho VT, Bassett IV et al. Sirolimus-based graft-versus-host disease prophylaxis protects against cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation: a cohort analysis. Blood 2007; 110: 490–500.
Couriel DR, Saliba R, Escalon MP, Hsu Y, Ghosh S, Ippoliti C et al. Sirolimus in combination with tacrolimus and corticosteroids for the treatment of resistant chronic graft-versus-host disease. Br J Haematol 2005; 130: 409–417.
Cutler C, Henry NL, Magee C, Li S, Kim HT, Alyea E et al. Sirolimus and thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2005; 11: 551–557.
Johnston LJ, Brown J, Shizuru JA, Stockerl-Goldstein KE, Stuart MJ, Blume KG et al. Rapamycin (sirolimus) for treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant 2005; 11: 47–55.
Cutler C, Stevenson K, Kim HT, Richardson P, Ho VT, Linden E et al. Sirolimus is associated with veno-occlusive disease of the liver after myeloablative allogeneic stem cell transplantation. Blood 2008; 112: 4425–4431.
Allison AC, Eugui EM . Mechanisms of action of mycophenolate mofetil in preventing acute and chronic allograft rejection. Transplantation 2005; 80 (2 Suppl): S181–S190.
Bestard O, Cruzado JM, Grinyo JM . Calcineurin-inhibitor-sparing immunosuppressive protocols. Transplant Proc 2005; 37: 3729–3732.
Ciancio G, Miller J, Gonwa TA . Review of major clinical trials with mycophenolate mofetil in renal transplantation. Transplantation 2005; 80 (2 Suppl): S191–S200.
Srinivas TR, Kaplan B, Schold JD, Meier-Kriesche HU . The impact of mycophenolate mofetil on long-term outcomes in kidney transplantation. Transplantation 2005; 80 (2 Suppl): S211–S220.
Basara N, Blau WI, Romer E, Rudolphi M, Bischoff M, Kirsten D et al. Mycophenolate mofetil for the treatment of acute and chronic GVHD in bone marrow transplant patients. Bone Marrow Transplant 1998; 22: 61–65.
Bornhauser M, Schuler U, Porksen G, Naumann R, Geissler G, Thiede C et al. Mycophenolate mofetil and cyclosporine as graft-versus-host disease prophylaxis after allogeneic blood stem cell transplantation. Transplantation 1999; 67: 499–504.
Kiehl MG, Schafer-Eckart K, Kroger M, Bornhauser M, Basara N, Blau IW et al. Mycophenolate mofetil for the prophylaxis of acute graft-versus-host disease in stem cell transplant recipients. Transplant Proc 2002; 34: 2922–2924.
Neumann F, Graef T, Tapprich C, Vaupel M, Steidl U, Germing U et al. Cyclosporine A and mycophenolate mofetil vs cyclosporine A and methotrexate for graft-versus-host disease prophylaxis after stem cell transplantation from HLA-identical siblings. Bone Marrow Transplant 2005; 35: 1089–1093.
Nash RA, Johnston L, Parker P, McCune JS, Storer B, Slattery JT et al. A phase I/II study of mycophenolate mofetil in combination with cyclosporine for prophylaxis of acute graft-versus-host disease after myeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2005; 11: 495–505.
Bolwell B, Sobecks R, Pohlman B, Andresen S, Rybicki L, Kuczkowski E et al. A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transplant 2004; 34: 621–625.
Nguyen VH, Zeiser R, Negrin RS . Role of naturally arising regulatory T cells in hematopoietic cell transplantation. Biol Blood Marrow Transplant 2006; 12: 995–1009.
Law LY, Horning SJ, Wong RM, Johnston LJ, Laport GG, Lowsky R et al. High-dose carmustine, etoposide, and cyclophosphamide followed by allogeneic hematopoietic cell transplantation for non-Hodgkin lymphoma. Biol Blood Marrow Transplant 2006; 12: 703–711.
Long GD, Amylon MD, Stockerl-Goldstein KE, Negrin RS, Chao NJ, Hu WW et al. Fractionated total-body irradiation, etoposide, and cyclophosphamide followed by allogeneic bone marrow transplantation for patients with high-risk or advanced-stage hematological malignancies. Biol Blood Marrow Transplant 1997; 3: 324–330.
Naik S, Wong R, Arai S, Brown J, Laport G, Lowsky R et al. Long-term outcomes in patients with high-risk myeloid malignancies following matched related donor hematopoietic cell transplantation with myeloablative conditioning of BU, etoposide and CY. Bone Marrow Transplant 2011; 46: 192–199.
Ho VT, Cutler C, Carter S, Martin P, Adams R, Horowitz M et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2005; 11: 571–575.
Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation 1987; 44: 778–783.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–956.
Giaccone L, McCune JS, Maris MB, Gooley TA, Sandmaier BM, Slattery JT et al. Pharmacodynamics of mycophenolate mofetil after nonmyeloablative conditioning and unrelated donor hematopoietic cell transplantation. Blood 2005; 106: 4381–4388.
Simon R . Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989; 10: 1–10.
Zeiser R, Negrin RS . Interleukin-2 receptor downstream events in regulatory T cells: implications for the choice of immunosuppressive drug therapy. Cell Cycle 2008; 7: 458–462.
Kahan BD, Podbielski J, Napoli KL, Katz SM, Meier-Kriesche HU, Van Buren CT . Immunosuppressive effects and safety of a sirolimus/cyclosporine combination regimen for renal transplantation. Transplantation 1998; 66: 1040–1046.
Kuppahally S, Al-Khaldi A, Weisshaar D, Valantine HA, Oyer P, Robbins RC et al. Wound healing complications with de novo sirolimus versus mycophenolate mofetil-based regimen in cardiac transplant recipients. Am J Transplant 2006; 6 (5 Part 1): 986–992.
Champion L, Stern M, Israel-Biet D, Mamzer-Bruneel MF, Peraldi MN, Kreis H et al. Brief communication: sirolimus-associated pneumonitis: 24 cases in renal transplant recipients. Ann Intern Med 2006; 144: 505–509.
Kahan BD . Sirolimus: a comprehensive review. Expert Opin Pharmacother 2001; 2: 1903–1917.
Groth CG, Backman L, Morales JM, Calne R, Kreis H, Lang P et al. Sirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Sirolimus European Renal Transplant Study Group. Transplantation 1999; 67: 1036–1042.
Kreis H, Cisterne JM, Land W, Wramner L, Squifflet JP, Abramowicz D et al. Sirolimus in association with mycophenolate mofetil induction for the prevention of acute graft rejection in renal allograft recipients. Transplantation 2000; 69: 1252–1260.
Kahan BD . Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group. Lancet 2000; 356: 194–202.
MacDonald AS . A worldwide, phase III, randomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation 2001; 71: 271–280.
Podder H, Stepkowski SM, Napoli KL, Clark J, Verani RR, Chou TC et al. Pharmacokinetic interactions augment toxicities of sirolimus/cyclosporine combinations. J Am Soc Nephrol 2001; 12: 1059–1071.
Furlong T, Kiem HP, Appelbaum FR, Carpenter PA, Deeg HJ, Doney K et al. Sirolimus in combination with cyclosporine or tacrolimus plus methotrexate for prevention of graft-versus-host disease following hematopoietic cell transplantation from unrelated donors. Biol Blood Marrow Transplant 2008; 14: 531–537.
Platzbecker U, von Bonin M, Goekkurt E, Radke J, Binder M, Kiani A et al. Graft-versus-host disease prophylaxis with everolimus and tacrolimus is associated with a high incidence of sinusoidal obstruction syndrome and microangiopathy: results of the EVTAC trial. Biol Blood Marrow Transplant 2009; 15: 101–108.
Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood 2006; 108: 390–399.
Strauss L, Czystowska M, Szajnik M, Mandapathil M, Whiteside TL . Differential responses of human regulatory T cells (Treg) and effector T cells to rapamycin. PLoS One 2009; 4: e5994.
Gatza E, Rogers CE, Clouthier SG, Lowler KP, Tawara I, Liu C et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood 2008; 112: 1515–1521.
Biagi E, Di Biaso I, Leoni V, Gaipa G, Rossi V, Bugarin C et al. Extracorporeal photochemotherapy is accompanied by increasing levels of circulating CD4+CD25+GITR+Foxp3+CD62L+ functional regulatory T-cells in patients with graft-versus-host disease. Transplantation 2007; 84: 31–39.
Rezvani K, Mielke S, Ahmadzadeh M, Kilical Y, Savani BN, Zeilah J et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood 2006; 108: 1291–1297.
Mielke S, Rezvani K, Savani BN, Nunes R, Yong AS, Schindler J et al. Reconstitution of FOXP3+ regulatory T cells (Tregs) after CD25-depleted allotransplantation in elderly patients and association with acute graft-versus-host disease. Blood 2007; 110: 1689–1697.
Jacobson P, Rogosheske J, Barker JN, Green K, Ng J, Weisdorf D et al. Relationship of mycophenolic acid exposure to clinical outcome after hematopoietic cell transplantation. Clin Pharmacol Ther 2005; 78: 486–500.
Jacobson P, Green K, Rogosheske J, Brunstein C, Ebeling B, DeFor T et al. Highly variable mycophenolate mofetil bioavailability following nonmyeloablative hematopoietic cell transplantation. J Clin Pharmacol 2007; 47: 6–12.
Golconda MS, Valente JF, Bejarano P, Gilinsky N, First MR . Mycophenolate mofetil-induced colonic ulceration in renal transplant recipients. Transplant Proc 1999; 31: 272–273.
Acknowledgements
This study was supported by the NIH Program Project Grant P01CA49605; Grant from the Doris Duke Charitable Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Johnston, L., Florek, M., Armstrong, R. et al. Sirolimus and mycophenolate mofetil as GVHD prophylaxis in myeloablative, matched-related donor hematopoietic cell transplantation. Bone Marrow Transplant 47, 581–588 (2012). https://doi.org/10.1038/bmt.2011.104
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2011.104
Keywords
This article is cited by
-
Uniform graft-versus-host disease prophylaxis with posttransplant cyclophosphamide, sirolimus, and mycophenolate mofetil following hematopoietic stem cell transplantation from haploidentical, matched sibling and unrelated donors
Bone Marrow Transplantation (2020)
-
Calcineurin inhibitor-free GVHD prophylaxis with sirolimus and mycophenolate mofetil combination
Annals of Hematology (2017)
-
Venous thromboembolism following hematopoietic stem cell transplantation—a systematic review and meta-analysis
Annals of Hematology (2016)
-
Pharmacokinetics, Pharmacodynamics, and Pharmacogenomics of Immunosuppressants in Allogeneic Hematopoietic Cell Transplantation: Part II
Clinical Pharmacokinetics (2016)
-
Mycophenolate mofetil-based salvage as acute GVHD prophylaxis after early discontinuation of tacrolimus and/or sirolimus
Bone Marrow Transplantation (2015)