Abstract
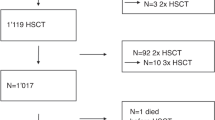
Four hundred and sixty-seven hematopoietic stem cell transplantations (HSCTs) (217 autologous and 250 allogeneic HSCT) were performed in 374 children at four pediatric HSCT centers in Korea from January 2005 to December 2007. Among 467 transplants, veno-occlusive disease (VOD) developed in 72 transplants (15.4%) at a median of 10 days after HSCT. Multivariate analysis showed that BU or TBI-containing regimen (P=0.002), VOD prophylaxis without lipo-prostaglandin E1 (PGE1) (P=0.012), number of previous HSCT (P=0.014), and pretransplant serum ferritin (P=0.018) were independent risk factors for developing VOD. Mean serum ferritin levels were significantly higher in HSCT with VOD (2109.6±2842.5 ng/ml) than in HSCT without VOD (1315.9±1094.4 ng/ml) (P<0.001). The relative risk of death within 100 days of HSCT in transplants with VOD compared with transplants without VOD was 3.39 (confidence interval: 1.78–6.45). Our results suggest that lipo-PGE1 might have a protective effect against the development of VOD, and pretransplant serum ferritin could act as a risk factor for VOD. A larger prospective study is needed to confirm a possible role of lipo-PGE1 and iron chelation therapy in reducing the incidence of VOD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ozkaynak MF, Weinberg K, Kohn D, Sender L, Parkman R, Lenarsky C . Hepatic veno-occlusive disease post-bone marrow transplantation in children conditioned with busulfan and cyclophosphamide: incidence, risk factors, and clinical outcome. Bone Marrow Transplant 1991; 7: 467–474.
Barker CC, Butzner JD, Anderson RA, Brant R, Sauve RS . Incidence, survival and risk factors for the development of veno-occlusive disease in pediatric hematopoietic stem cell transplant recipients. Bone Marrow Transplant 2003; 32: 79–87.
Cesaro S, Pillon M, Talenti E, Toffolutti T, Calore E, Tridello G et al. A prospective survey on incidence, risk factors and therapy of hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation. Haematologica 2005; 90: 1396–1404.
Cacchione A, LeMaitre A, Couanet DV, Benhamou E, Amoroso L, Simonnard N et al. Risk factors for hepatic veno-occlusive disease: a retrospective unicentric study in 116 children autografted after a high-dose BU-thiotepa regimen. Bone Marrow Transplant 2008; 42: 449–454.
Shulman HM, Hinterberger W . Hepatic veno-occlusive disease—liver toxicity syndrome after bone marrow transplantation. Bone Marrow Transplant 1992; 10: 197–214.
Hasegawa S, Horibe K, Kawabe T, Kato K, Kojima S, Matsuyama T et al. Veno-occlusive disease of the liver after allogeneic bone marrow transplantation in children with hematologic malignancies: incidence, onset time and risk factors. Bone Marrow Transplant 1998; 22: 1191–1197.
Radich JP, Sanders JE, Buckner CD, Martin PJ, Petersen FB, Bensinger W et al. Second allogeneic marrow transplantation for patients with recurrent leukemia after initial transplant with total-body irradiation-containing regimens. J Clin Oncol 1993; 11: 304–313.
Carreras E, Bertz H, Arcese W, Vernant JP, Tomás JF, Hagglund H et al. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European Group for Blood and Marrow Transplantation. European Group for Blood and Marrow Transplantation Chronic Leukemia Working Party. Blood 1998; 92: 3599–3604.
Wadleigh M, Richardson PG, Zahrieh D, Lee SJ, Cutler C, Ho V et al. Prior gemtuzumab ozogamicin exposure significantly increases the risk of veno-occlusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood 2003; 102: 1578–1582.
Valteau-Couanet D, Benhamou E, Vassal G, Stambouli F, Lapierre V, Couanet D et al. Consolidation with a busulfan-containing regimen followed by stem cell transplantation in infants with poor prognosis stage 4 neuroblastoma. Bone Marrow Transplant 2000; 25: 937–942.
Méresse V, Hartmann O, Vassal G, Benhamou E, Valteau-Couanet D, Brugieres L et al. Risk factors for hepatic veno-occlusive disease after high-dose busulfan-containing regimens followed by autologous bone marrow transplantation: a study in 136 children. Bone Marrow Transplant 1992; 10: 135–141.
Corbacioglu S, Hönig M, Lahr G, Stöhr S, Berry G, Friedrich W et al. Stem cell transplantation in children with infantile osteopetrosis is associated with a high incidence of VOD, which could be prevented with defibrotide. Bone Marrow Transplant 2006; 38: 547–553.
Attal M, Huguet F, Rubie H, Huynh A, Charlet JP, Payen JL et al. Prevention of hepatic veno-occlusive disease after bone marrow transplantation by continuous infusion of low-dose heparin: a prospective, randomized trial. Blood 1992; 79: 2834–2840.
Bearman SI, Hinds MS, Wolford JL, Petersen FB, Nugent DL, Slichter SJ et al. A pilot study of continuous infusion of heparin for the prevention of hepatic veno-occlusive disease after bone marrow transplantation. Bone Marrow Transplant 1990; 5: 407–411.
Marsa-Vila L, Gorin NC, Laporte JP, Labopin M, Dupuy-Montbrun MC, Fouillard L et al. Prophylactic heparin does not prevent liver veno-occlusive disease following autologous bone marrow transplantation. Eur J Haematol 1991; 47: 346–354.
Simon M, Hahn T, Ford LA, Anderson B, Swinnich D, Baer MR et al. Retrospective multicenter analysis of hepatic veno-occlusive disease after blood or marrow transplantation: possible beneficial use of low molecular weight heparin. Bone Marrow Transplant 2001; 27: 627–633.
Forrest DL, Thompson K, Dorcas VG, Couban SH, Pierce R . Low molecular weight heparin for the prevention of hepatic veno-occlusive disease (VOD) after hematopoietic stem cell transplantation: a prospective phase II study. Bone Marrow Transplant 2003; 31: 1143–1149.
Gluckman E, Jolivet I, Scrobohaci ML, Devergie A, Traineau R, Bourdeau-Esperou H et al. Use of prostaglandin E1 for prevention of liver veno-occlusive disease in leukaemic patients treated by allogeneic bone marrow transplantation. Br J Haematol 1990; 74: 277–281.
Song JS, Seo JJ, Moon HN, Ghim T, Im HJ . Prophylactic low-dose heparin or prostaglandin E1 may prevent severe veno-occlusive disease of the liver after allogeneic hematopoietic stem cell transplantation in Korean children. J Korean Med Sci 2006; 21: 897–903.
Chalandon Y, Roosnek E, Mermillod B, Newton A, Ozsahin H, Wacker P et al. Prevention of veno-occlusive disease with defibrotide after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2004; 10: 347–354.
Dignan F, Gujral D, Ethell M, Evans S, Treleaven J, Morgan G et al. Prophylactic defibrotide in allogeneic stem cell transplantation: minimal morbidity and zero mortality from veno-occlusive disease. Bone Marrow Transplant 2007; 40: 79–82.
Qureshi A, Marshall L, Lancaster D . Defibrotide in the prevention and treatment of veno-occlusive disease in autologous and allogeneic stem cell transplantation in children. Pediatr Blood Cancer 2008; 30: 831–832.
McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 1993; 118: 255–267.
Brugieres L, Hartmann O, Benhamou E, Zafrani ES, Caillaud JM, Patte C et al. Veno-occlusive disease of the liver following high-dose chemotherapy and autologous bone marrow transplantation in children with solid tumors: incidence, clinical course and outcome. Bone Marrow Transplant 1988; 3: 53–58.
Bearman SI, Anderson GL, Mori M, Hinds MS, Shulman HM, McDonald GB . Venoocclusive disease of the liver: development of a model for predicting fatal outcome after marrow transplantation. J Clin Oncol 1993; 11: 1729–1736.
Toh HC, McAfee SL, Sackstein R, Cox BF, Colby C, Spitzer TR . Late onset veno-occlusive disease following high-dose chemotherapy and stem cell transplantation. Bone Marrow Transplant 1999; 24: 891–895.
Styler MJ, Crilley P, Biggs J, Moul J, Copelan E, Topolsky D et al. Hepatic dysfunction following busulfan and cyclophosphamide myeloablation: a retrospective, multicenter analysis. Bone Marrow Transplant 1996; 18: 171–176.
Girinsky T, Benhamou E, Bourhis JH, Dhermain F, Guillot-Valls D, Ganansia V et al. Prospective randomized comparison of single-dose versus hyperfractionated total-body irradiation in patients with hematologic malignancies. J Clin Oncol 2000; 18: 981–986.
Cosset JM, Baume D, Pico JL, Shank B, Girinski T, Benhamou E et al. Single dose versus hyperfractionated total body irradiation before allogeneic bone marrow transplantation: a non-randomized comparative study of 54 patients at the Institute Gustave-Roussy. Radiother Oncol 1989; 15: 151–160.
Grochow LB, Jones RJ, Brundrett RB, Braine HG, Chen TL, Saral R et al. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol 1989; 25: 55–61.
Cutting R, Mirelman A, Vora A . Treosulphan as an alternative to busulphan for myeloablative conditioning in paediatric allogeneic transplantation. Br J Haematol 2008; 143: 748–751.
Beelen DW, Trenschel R, Casper J, Freund M, Hilger RA, Scheulen ME et al. Dose-escalated treosulphan in combination with cyclophosphamide as a new preparative regimen for allogeneic haematopoietic stem cell transplantation in patients with an increased risk for regimen-related complications. Bone Marrow Transplant 2005; 35: 233–241.
Bearman SI, Shen DD, Hinds MS, Hill HA, McDonald GB . A phase I/II study of prostaglandin E1 for the prevention of hepatic venocclusive disease after bone marrow transplantation. Br J Haematol 1993; 84: 724–730.
Piper PJ, Vane JR, Wyllie JH . Inactivation of prostaglandins by the lungs. Nature 1970; 225: 600–604.
Mizushima Y, Yanagawa A, Hoshi K . Prostaglandin E1 is more effective, when incorporated in lipid microspheres, for treatment of peripheral vascular diseases in man. J Pharm Pharmacol 1983; 35: 666–667.
Sim AK, McCraw AP, Cleland ME, Aihara H, Otomo S, Hosoda K . The effect of prostaglandin E1 incorporated in lipid microspheres on thrombus formation and thrombus disaggregation and its potential to target to the site of vascular lesions. Arzneimittelforschung 1986; 36: 1206–1209.
Otomo S, Mizushima Y, Aihara H, Yokoyama K, Watanabe M, Yanagawa A . Prostaglandin E1 incorporated in lipid microspheres (lipo PGE1). Drugs Exp Clin Res 1985; 11: 627–631.
Mizushima Y, Hamano T, Haramoto S, Kiyokawa S, Yanagawa A, Nakura K et al. Distribution of lipid microspheres incorporating prostaglandin E1 to vascular lesions. Prostaglandins Leukot Essent Fatty Acids 1990; 41: 269–272.
Evens AM, Mehta J, Gordon LI . Rust and corrosion in hematopoietic stem cell transplantation: the problem of iron and oxidative stress. Bone Marrow Transplant 2004; 34: 561–571.
Olivieri NF, Brittenham GM, McLaren CE, Templeton DM, Cameron RG, McClelland RA et al. Long-term safety and effectiveness of iron-chelation therapy with deferiprone for thalassemia major. N Engl J Med 1998; 339: 417–423.
Olivieri NF, Nathan DG, MacMillan JH, Wayne AS, Liu PP, McGee A et al. Survival in medically treated patients with homozygous beta-thalassemia. N Engl J Med 1994; 331: 574–578.
Morado M, Ojeda E, Garcia-Bustos J, Aguado MJ, Arrieta R, Quevedo E et al. BMT: serum ferritin as risk factor for veno-occlusive disease of the liver. Prospective Cohort Study. Hematology 2000; 4: 505–512.
Armand P, Kim HT, Cutler CS, Ho VT, Koreth J, Alyea EP et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood 2007; 109: 4586–4588.
Jastaniah W, Harmatz P, Pakbaz Z, Fischer R, Vichinsky E, Walters MC . Transfusional iron burden and liver toxicity after bone marrow transplantation for acute myelogenous leukemia and hemoglobinopathies. Pediatr Blood Cancer 2008; 50: 319–324.
Acknowledgements
This study was supported by a Korea Childhood Leukemia Foundation, by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health, Welfare and Family affairs, Republic of Korea (A080588) and by a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family affairs, Republic of Korea (0520290).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lee, S., Yoo, K., Sung, K. et al. Hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation: incidence, risk factors, and outcome. Bone Marrow Transplant 45, 1287–1293 (2010). https://doi.org/10.1038/bmt.2009.349
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2009.349
Keywords
This article is cited by
-
Hypofibrinolysis in pediatric patients with veno-occlusive disease in hematopoietic stem cell transplantation
Journal of Cancer Research and Clinical Oncology (2023)
-
Incidence and risk factors of hepatic veno-occlusive disease/sinusoidal obstruction syndrome after allogeneic hematopoietic cell transplantation in adults with prophylactic ursodiol and intravenous heparin or prostaglandin E1
Bone Marrow Transplantation (2021)
-
Hepatic veno-occlusive disease/sinusoidal obstruction syndrome after hematopoietic stem cell transplantation for thalassemia major: incidence, management, and outcome
Bone Marrow Transplantation (2021)
-
Introduction of new pediatric EBMT criteria for VOD diagnosis: is it time-saving or money-wasting?
Bone Marrow Transplantation (2020)
-
Validation of treatment outcomes according to revised severity criteria from European Society for Blood and Marrow Transplantation (EBMT) for sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD)
Bone Marrow Transplantation (2019)