Abstract
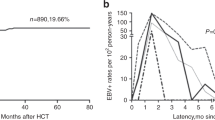
EBV-associated post transplant lymphoproliferative disease (EBV-PTLD) is a life-threatening complication that may occur after hemopoietic SCT. We prospectively screened 80 children on a weekly basis using nested quantitative PCR to evaluate EBV genome copies. EBV viral load <1000 copies per 105 PBMC was observed in 63% of transplants, whereas it was between 1000 and 9999 copies per 105 PBMC in 13%, and between 10 000 and 19 999 in 10%, with no significant increase in percentage of CD20+ lymphocytes. Viral load reached ⩾20 000 copies per 105 PBMC in 14% of patients, and rituximab was administered to 75% of them. None of the patients except one developed a lymphoproliferative disease. Our study found that only 13% of unrelated donor HSCT recipients had a very high risk of EBV-PTLD defined as ⩾20 000 geq per 105 PBMC associated with an increase in CD20+ lymphocyte. We suggest that rituximab could be administered in the presence of very high levels of EBV-DNA viral load or in the presence of mid levels of EBV-DNA viral load associated with an increase in the percentage of CD20+ lymphocytes. Through this approach, we significantly reduced the number of patients treated with rituximab, and consequently the acute and chronic adverse events related to this treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Styczynski J, Einsele H, Gil L, Ljungman P . Outcome of treatment of Epstein–Barr virus-related post-transplant lymphoproliferative disorder in hematopoietic stem cell recipients: a comprehensive review of reported cases. Transpl Infect Dis 2009; 11: 383–392.
Bar-Natan M, Nagler A . Epstein–Barr virus-associated post-transplant lymphoproliferative disorder. Isr Med Assoc J 2006; 8: 205–207.
Ocheni S, Kroeger N, Zabelina T, Sobottka I, Ayuk F, Wolschke C et al. EBV reactivation and post transplant lymphoproliferative disorders following allogeneic SCT. Bone Marrow Transplant 2008; 42: 181–186.
van Esser JW, Niesters HG, van der Holt B, Meijer E, Osterhaus AD, Gratama JW et al. Prevention of Epstein–Barr virus-lymphoproliferative disease by molecular monitoring and preemptive rituximab in high-risk patients after allogeneic stem cell transplantation. Blood 2002; 99: 4364–4369.
Curtis RE, Travis LB, Rowlings PA, Socie G, Kingma DW, Banks PM et al. Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood 1999; 94: 2208–2216.
Tsao L, Hsi ED . The clinicopathologic spectrum of posttransplantation lymphoproliferative disorders. Arch Pathol Lab Med 2007; 131: 1209–1218.
Gruhn B, Meerbach A, Hafer R, Zell R, Wutzler P, Zintl F . Pre-emptive therapy with rituximab for prevention of Epstein–Barr virus-associated lymphoproliferative disease after hematopoietic stem cell transplantation. Bone Marrow Transplant 2003; 31: 1023–1025.
Lucas KG, Burton RL, Zimmerman SE, Wang J, Cornetta KG, Robertson KA et al. Semiquantitative Epstein–Barr virus (EBV) polymerase chain reaction for the determination of patients at risk for EBV-induced lymphoproliferative disease after stem cell transplantation. Blood 1998; 91: 3654–3661.
Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein–Barr virus-induced lymphoma in allogeneic transplant recipients. Blood 1998; 92: 1549–1555.
Lenz HJ . Management and preparedness for infusion and hypersensitivity reactions. Oncologist 2007; 12: 601–609.
Biehn SE, Kirk D, Rivera MP, Martinez AE, Khandani AH, Orlowski RZ . Bronchiolitis obliterans with organizing pneumonia after rituximab therapy for non-Hodgkin's lymphoma. Hematol Oncol 2006; 24: 234–237.
Castagnola E, Dallorso S, Faraci M, Morreale G, Di Martino D, Cristina E et al. Long-lasting hypogammaglobulinemia following rituximab administration for Epstein–Barr virus-related post-transplant lymphoproliferative disease preemptive therapy. J Hematother Stem Cell Res 2003; 12: 9–10.
Greenfield HM, Gharib MI, Turner AJ, Guiver M, Carr T, Will AM et al. The impact of monitoring Epstein–Barr virus PCR in paediatric bone marrow transplant patients: can it successfully predict outcome and guide intervention? Pediatr Blood Cancer 2006; 47: 200–205.
Imashuku S, Teramura T, Morimoto A, Naya M, Kuroda H . Prolonged hypogammaglobulinemia following rituximab treatment for post transplant Epstein–Barr virus-associated lymphoproliferative disease. Bone Marrow Transplant 2004; 33: 129–130.
Castagnola E, Bagnasco F, Faraci M, Caviglia I, Caruso S, Cappelli B et al. Incidence of bacteremias and invasive mycoses in children undergoing allogeneic hematopoietic stem cell transplantation: a single center experience. Bone Marrow Transplant 2008; 41: 339–347.
Faraci M, Lanino E, Micalizzi C, Morreale G, Di Martino D, Banov L et al. Unrelated hematopoietic stem cell transplantation for Cernunnos-XLF deficiency. Pediatr Transplant 2009; 13: 785–789.
Styczynski J, Reusser P, Einsele H, de la Camara R, Cordonnier C, Ward KN et al. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant 2009; 43: 757–770.
van Esser JW, van der Holt B, Meijer E, Niesters HG, Trenschel R, Thijsen SF et al. Epstein–Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and quantitatively predicts EBV-lymphoproliferative disease following T-cell-depleted SCT. Blood 2001; 98: 972–978.
Kinch A, Oberg G, Arvidson J, Falk KI, Linde A, Pauksens K . Post-transplant lymphoproliferative disease and other Epstein–Barr virus diseases in allogeneic haematopoietic stem cell transplantation after introduction of monitoring of viral load by polymerase chain reaction. Scand J Infect Dis 2007; 39: 235–244.
Cesaro S, Murrone A, Mengoli C, Pillon M, Biasolo MA, Calore E et al. The real-time polymerase chain reaction-guided modulation of immunosuppression enables the pre-emptive management of Epstein–Barr virus reactivation after allogeneic haematopoietic stem cell transplantation. Br J Haematol 2005; 128: 224–233.
Rowe DT, Qu L, Reyes J, Jabbour N, Yunis E, Putnam P et al. Use of quantitative competitive PCR to measure Epstein–Barr virus genome load in the peripheral blood of pediatric transplant patients with lymphoproliferative disorders. J Clin Microbiol 1997; 35: 1612–1615.
Meerbach A, Wutzler P, Hafer R, Zintl F, Gruhn B . Monitoring of Epstein–Barr virus load after hematopoietic stem cell transplantation for early intervention in post-transplant lymphoproliferative disease. J Med Virol 2008; 80: 441–454.
Ahmad I, Cau NV, Kwan J, Maaroufi Y, Meuleman N, Aoun M et al. Preemptive management of Epstein–Barr virus reactivation after hematopoietic stem-cell transplantation. Transplantation 2009; 87: 1240–1245.
Aalto SM, Juvonen E, Tarkkanen J, Volin L, Haario H, Ruutu T et al. Epstein–Barr viral load and disease prediction in a large cohort of allogeneic stem cell transplant recipients. Clin Infect Dis 2007; 45: 1305–1309.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Faraci, M., Caviglia, I., Morreale, G. et al. Viral-load and B-lymphocyte monitoring of EBV reactivation after allogeneic hemopoietic SCT in children. Bone Marrow Transplant 45, 1052–1055 (2010). https://doi.org/10.1038/bmt.2009.302
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2009.302
Keywords
This article is cited by
-
Combination of immunoglobulins and natural killer cells in the context of CMV and EBV infection
Medical Microbiology and Immunology (2014)
-
Reduced PTLD-related mortality in patients experiencing EBV infection following allo-SCT after the introduction of a protocol incorporating pre-emptive rituximab
Bone Marrow Transplantation (2013)
-
Molecular Diagnosis and Management of Viral Infections in Hematopoietic Stem Cell Transplant Recipients
Molecular Diagnosis & Therapy (2012)