Abstract
Background:
Use of antibiotics could alter human microbiota composition and decrease bacterial diversity. Such microbial dysbiosis may have implications in hepatocarcinogenesis; however, the association between antibiotic use and liver cancer risk has been minimally examined in humans.
Methods:
We performed a nested case–control study (1195 primary liver cancer cases and 4640 matched controls) within the United Kingdom’s Clinical Practice Research Datalink. Antibiotic use was obtained from prescription records. Multivariable-adjusted odds ratio (OR) and 95% confidence interval (CI) were estimated using conditional logistic regression.
Results:
Ever-use of prescription antibiotics was associated with a slightly increased risk of liver cancer, compared to non-use (OR=1.22, 95% CI=1.03−1.45). However, there was no clear dose–response relationship by the number of prescriptions or cumulative dose of antibiotic use, suggesting a non-causal association.
Conclusions:
Our results do not support a role of antibiotic use in liver cancer development.
Similar content being viewed by others
Main
Antibiotics are widely used to treat or prevent bacterial infection. However, they can also disturb the normal composition of gut microbiota, resulting in microbial dysbiosis including decreased bacterial diversity and increased antibiotic-resistant pathogens (Clemente et al, 2012). Such dysbiosis could increase hepatic exposure to cancer-promoting microbial products and metabolites that reach the liver through the portal vein (Schwabe and Jobin, 2013). Thus, we hypothesised that antibiotic use may be associated with increased risk of liver cancer. To our knowledge, only two population-based studies have investigated this hypothesis, with inconsistent results (Kilkkinen et al, 2008; Boursi et al, 2015). It is important to expand this evidence base due to the wide use of antibiotics in clinical settings.
Herein, we investigated the association of prescription antibiotic use with the risk of primary liver cancer within the Clinical Practice Research Datalink (CPRD) in the UK.
Materials and Methods
Data source
This nested case–control study was based in the CPRD, a large, population-based, electronic medical record database with information on ∼8.5% of the UK population (Jick et al, 1991; Lawson et al, 1998; Jick et al, 2003). This study was approved by the National Institutes of Health Human Research Protection Program and the Independent Scientific Advisory Committee of the CPRD (Protocol 12_127R2A).
Study population
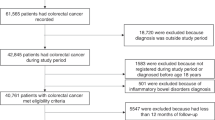
Cases and controls were drawn from persons enrolled in the CPRD from 1988 through 2011 who were between the ages of 10 and 90 years. Cases met the following criteria: (1) had a first time diagnosis of primary liver cancer, (2) had no prior diagnosis of cancers most likely to metastasise to the liver (lung, stomach, breast, colon, or pancreatic cancer) and no code of liver metastases, and (3) had no diagnosis of any other cancer (except for non-melanoma skin cancer) in 3 years before the index date. The index date of cases was defined as 1 year before liver cancer diagnosis, and all cases were required to have at least 2 years of history in the CPRD before the index date. For each case, controls were selected from individuals who were in the CPRD at the case’s index date and had no cancer diagnosis (except non-melanoma skin cancer) before that date. Controls were matched to cases at a four-to-one ratio on age (same year of birth), sex, general practice, and number of years in the CPRD before the case’s index date. We then defined the controls’ index date to be the same as the matched case’s index date. We only identified three eligible controls for 59 of the cases, two for 24 cases, and one for 11 cases, thus the number of controls totalled 4640.
In addition to the full case–control match, we completed an additional match based on the presence of diabetes, at a four-to-one ratio using the same matching factors as in the primary match. Overall, 1379 controls with diabetes were matched to the 346 cases with diabetes and 3396 controls without diabetes were matched to the 849 cases without diabetes.
Exposure definition
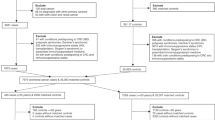
We identified all antibiotic prescriptions (Supplementary Table 1) recorded before the index date from the electronic records. Ever-use of antibiotics was defined as having two or more antibiotic prescriptions before the index date, and non-use was defined as having none or one prescription before the index date. We additionally examined antibiotic use by the total number of prescriptions, cumulative dose (the number of pills multiplied by the dose per pill, summed from first entry into CPRD through the index date), and recency of use (current use was defined as use that ended within 1 year before the index date, whereas past use was defined as use that ended more than 1 year before the index date). Furthermore, to assess the intensity of antibiotic use, we calculated the time between the first and last use (categorised as <2 years, 2–5 years, and >5 years) and examined the association between total number of prescriptions and liver cancer risk within each time period category.
Statistical analysis
We used conditional logistic regression to calculate the crude and adjusted odds ratio (OR) and 95% confidence intervals (CI). We adjusted for the following factors in multivariable models selected a priori based on previous literature: body mass index (BMI), smoking status, alcohol-related disorders, hepatitis B or C virus (HBV or HCV) infection, diabetes, rare metabolic disorders, and use of anti-diabetic medications, paracetamol, and statins. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
As shown in Table 1, cases (n=1195) were more likely to be obese, be current or former smokers, have HBV and/or HCV infection, chronic liver disease, rare metabolic disorders, alcohol-related disorders, or diabetes, and to take anti-diabetic medication or paracetamol, compared to their matched controls (n=4640).
Table 2 shows that ever-use of antibiotics was associated with a 22% higher risk of liver cancer, compared to non-use (OR=1.22, 95% CI: 1.03−1.45). However, there was no clear dose–response relationship by the number of prescriptions or by cumulative dose. When we examined major classes of antibiotics, the inhibitors of nucleic acid synthesis yielded the highest OR, but sample size was limited (Supplementary Table 2). In analyses based on additional matching according to the presence of diabetes, antibiotic use was associated with increased risk of liver cancer in both the diabetes match and the non-diabetes match (Table 3).
None of the results materially changed when we conducted the following sensitivity analyses: (1) using an index date of 2 years before the case’s date of diagnosis, rather than 1 year; (2) restricting to patients with 5 or more years of information in their medical record before the index date; and (3) using 0 prescriptions (rather than 0–1 prescriptions) as the reference group.
Discussion
In this nested case–control study, we observed an OR of 1.22 (95% CI: 1.03−1.45) of liver cancer for antibiotic ever-use compared to non-use. Our results do not support a causal association as there was no clear pattern of dose–response by the number of prescriptions or cumulative dose.
Before our study, only two population-based studies have examined the association of antibiotic use and risk of liver cancer, with inconsistent results (Kilkkinen et al, 2008; Boursi et al, 2015). In our study, ever-use of prescription antibiotics was associated with slightly increased risk of liver cancer. One possible mechanism underlying this association is antibiotic-induced disturbance of commensal microbiota and subsequent dysbiosis, which may result in increased hepatic exposure to bacterial products and metabolites that could be carcinogenic (Schwabe and Jobin, 2013). Alternatively, given the lack of dose–response, confounding may at least partially explain the slightly elevated risk of liver cancer in our study. Several important risk factors of liver cancer, such as cirrhosis and diabetes, are associated with increased risk of bacterial infection (Navasa et al, 1997; Joshi et al, 1999), which may subsequently require antibiotic treatments. Thus, liver cancer cases may have used antibiotics to treat bacterial infections that arose from these clinical conditions before cancer diagnosis. To explore this possibility, we created a relatively ‘clean’ population using the non-diabetes match, and further excluded individuals with chronic liver disease; however the results were similar to what we observed in the main analysis (OR 1.23, 95% CI: 0.99–1.51). It is possible that among this relatively ‘clean’ population, residual confounding by other unmeasured variables still exists. Thus, whether the slightly elevated risk of liver cancer in our study is explained by confounding warrants further investigation.
A major strength of this study is that the analysis was conducted using the CPRD, a large, well-established, validated, longitudinal primary-care database known for diagnostic accuracy of cancer outcomes and complete outpatient prescription pharmaceutical data. Antibiotic use was obtained from prescription records before cancer diagnosis, which minimised recall bias. A possible limitation is that liver cancer diagnosis was not confirmed by linkage to a cancer registry; previous validation studies have shown that cancer diagnoses within the CPRD are reasonably complete (Jick et al, 1991), however we cannot rule out the possibility that a proportion of liver cancer diagnoses were missed. As discussed above, we do not have comprehensive information on risk factors of liver cancer that may require antibiotic treatments; thus we are unable to sufficiently evaluate whether the observed excess risk was due to residual confounding. In addition, prescription records may not reflect the actual usage of antibiotics, and antibiotic use before entry into CPRD was unavailable, thus exposure misclassification is possible.
In conclusion, our results do not support a role of antibiotics in the development of liver cancer.
Change history
28 June 2016
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Boursi B, Mamtani R, Haynes K, Yang Y-X (2015) Recurrent antibiotic exposure may promote cancer formation—another step in understanding the role of the human microbiota? Eur J Cancer 51 (17): 2655–2664.
Clemente Jose C, Ursell Luke K, Parfrey Laura W, Knight R (2012) The impact of the gut microbiota on human health: an integrative view. Cell 148 (6): 1258–1270.
Jick H, Jick SS, Derby LE (1991) Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ 302 (6779): 766–768.
Jick SS, Kaye JA, Vasilakis-Scaramozza C, Rodríguez LAG, Ruigómez A, Meier CR, Schlienger RG, Black C, Jick H (2003) Validity of the general practice research database. Pharmacotherapy 23 (5): 686–689.
Joshi N, Caputo GM, Weitekamp MR, Karchmer AW (1999) Infections in patients with diabetes mellitus. N Engl J Med 341 (25): 1906–1912.
Kilkkinen A, Rissanen H, Klaukka T, Pukkala E, Heliövaara M, Huovinen P, Männistö S, Aromaa A, Knekt P (2008) Antibiotic use predicts an increased risk of cancer. Int J Cancer 123 (9): 2152–2155.
Lawson DH, Sherman V, Hollowell J (1998) The General Practice Research Database. Scientific and Ethical Advisory Group. QJM 91 (6): 445–452.
Navasa M, Rimola A, Rodés J (1997) Bacterial infections in liver disease. Semin Liver Dis 17 (4): 323–333.
Schwabe RF, Jobin C (2013) The microbiome and cancer. Nat Rev Cancer 13 (11): 800–812.
Acknowledgements
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute. We thank Ms. Megan Braunlin for her help with the analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Yang, B., Hagberg, K., Chen, J. et al. Associations of antibiotic use with risk of primary liver cancer in the Clinical Practice Research Datalink. Br J Cancer 115, 85–89 (2016). https://doi.org/10.1038/bjc.2016.148
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2016.148
Keywords
This article is cited by
-
Mechanisms by which the intestinal microbiota affects gastrointestinal tumours and therapeutic effects
Molecular Biomedicine (2023)
-
Early life gut microbiota sustains liver-resident natural killer cells maturation via the butyrate-IL-18 axis
Nature Communications (2023)