Abstract
Background:
We evaluated the efficacy and safety of the modified FOLFOX6 (mFOLFOX6) regimen as a neoadjuvant chemotherapy in gastric cancer patients.
Methods:
Seventy-three patients with T2–T4 or N+ were enroled. Preoperative chemotherapy consisted of three cycles of mFOLFOX6. The primary end points were the response rate and the R0 resection rate. Prognostic factors for overall survival (OS) were investigated using univariate and multivariate analyses.
Results:
Sixty-seven (91.8%) patients completed 3 cycles, with grade 3–4 toxicity arising in 33.0%. The radiology response rate was 45.8%. Sixty-seven (91.8%) patients receiving radical surgery showed different levels of histological regression of the primary tumour, with a ⩾50% regression rate of 49.2%. ypTNM stage (HR 4.045, 95% CI 1.429–11.446) and tumours of diffuse and mixed type (HR 9.963, 95% CI 1.937–51.235; HR 8.890, 95% CI 1.157–68.323, respectively) were significantly associated with OS. The pathologic regression rate (GHR; ⩾2/3/<2/3, ⩾50%/<50%) was statistically significantly associated with OS according to a univariate analysis.
Conclusions:
Perioperative mFOLFOX6 was a tolerable and effective regimen for gastric cancer. The ypTNM stage was an independent predictor of survival. GHR ⩾50%/<50% could be used as a surrogate marker for selecting a postoperative chemotherapy regimen.
Similar content being viewed by others
Main
Since Wilke et al (1989) first reported the use of neoadjuvant chemotherapy for treating gastric cancer in 1989, the potential benefits of downstaging the primary tumour, facilitating complete surgical resection and treating systemic micrometastases have received much attention. Especially in recent years, clinical research (Cunningham et al, 2006; Lutz et al, 2012; Ychou et al, 2011) has indicated that preoperative chemotherapy for gastric cancer (neoadjuvant chemotherapy) could improve the R0 resection rate in surgery for advanced gastric cancer and overall survival (OS). The Medical Research Council Adjuvant Gastric Infusion Chemotherapy (MAGIC) trial published in 2006 (Cunningham et al, 2006) showed that perioperative chemotherapy could increase the 5-year survival rate from 23 to 36%; the difference in the rates of postoperative complications between the surgery group and the perioperative-chemotherapy group was not statistically significant. Hence, since 2008, the National Comprehensive Cancer Network (NCCN) Guideline has recommended a regimen of epirubicin, cisplatin and infused fluorouracil (ECF) as the standard neoadjuvant approach for T2 and higher staging of gastric cancer (NCCN Clinical Practice Guidelines in Oncology, 2007). Li et al (2010) published a meta-analysis that included 2271 patients with advanced gastric cancer, and the results indicated that neoadjuvant chemotherapy could improve the tumour stage, increase the R0 resection rate and provide survival benefits for patients with locally advanced gastric cancer (OR=1.27, 95% confidence interval:1.04–1.55). However, these clinical studies included different neoadjuvant chemotherapy regimens and operative approaches; therefore, it is still unclear which chemotherapy regimen is the most effective.
Another important role of neoadjuvant chemotherapy is to evaluate the effect of the neoadjuvant chemotherapy regimen to guide the selection of the postoperative chemotherapy regimen. However, which method can best evaluate the effect of preoperative chemotherapy, and which standard should be used to determine whether a change in postoperative therapies is required, are the key issues and are still controversial. The best approach is to observe the impact of neoadjuvant chemotherapy on the recurrence and survival rates. However, because both operative approaches and postoperative therapy regimens influence the final outcome of treatments, it is difficult to accurately assess the impact of preoperative chemotherapy on survival outcomes; therefore, histologic and imaging response assessments are the most direct and convenient predictive methods of neoadjuvant chemotherapy. The methods for evaluating the response rate by the Response Evaluation Criteria in Solid Tumors (Husband et al, 2004) and histological response described by the Japanese Gastric Cancer Association (2011) (JCGC) have been used to evaluate the effect of neoadjuvant chemotherapy. The correlation between the grade of histologic regression and prognosis has been used in the evaluation of various tumours, including pancreatic cancer, rectal cancer and oesophageal cancer, but the prognostic value of this variable has been mixed and is still controversial in advanced gastric cancer (Lowy et al, 1999; Breslin et al, 2001; Onaitis et al, 2001; Ruo et al, 2002; Berger et al, 2005; Chirieac et al, 2005; Brenner et al, 2006; Gaca et al, 2006; Gu et al, 2006; Mansour et al, 2007).
Therefore, a prospective study was performed in the Department of General Surgery, Peking Union Medical College Hospital, from December 2006 to October 2012. A total of 73 patients were enroled, and the modified FOLFOX6 (mFOLFOX6) was adopted as the regimen of neoadjuvant chemotherapy.
The aims of the current study were as follows: (1) to analyse the efficacy and safety of the mFOLFOX6 regimen in locally advanced gastric cancer patients and (2) to investigate whether a histologic response or radiographic response was a superior surrogate marker for OS, which can be used to determine the postoperative chemotherapy regimen.
Materials and Methods
Patients
The inclusion criteria were as follows: (1) histologically confirmed gastric or gastroesophageal junction carcinoma; (2) TNM stage of T2–T4 or positive regional lymph nodes according to the AJCC 7.0 staging system, verified by enhanced computed tomography (CT), with no evidence of distant metastases; (3) ECOG performance status score ⩽0–2 and adequate haematological, heart, liver and renal functions; (4) over 18 years old; (5) never received any chemotherapy, radiotherapy or surgical treatments for gastric cancer; and (6) signed the informed consent form.
The exclusion criteria were as follows: (1) women in a gestation or lactation period, (2) presence of infectious diseases, (3) watery diarrhoea, (4) complications involving peripheral neuropathy, (5) active bleeding of the gastrointestinal tract, (6) complications involving tumours in other sites except for preinvasive carcinoma and (7) sensitivity to investigational drugs or iodines.
The patients were enroled, as we previously described, after providing informed consent according to procedures approved by the Ethics Committee at Peking Union Medical College hospital. The study was approved by the Ethics Committee of Peking Union Medical College Hospital and registered on the ClinicalTrails.gov website (registration ID: NCT02226380).
Treatment
The enroled patients were treated with a mFOLFOX6 regimen: a 2-h intravenous injection of oxaliplatin (85 mg m−2) on day 1, a 2-h intravenous injection of leucovorin (400 mg m−2) on day 1, an intravenous injection of 5-FU (400 mg m−2), and then a 46-h continuous infusion (2400 mg m−2) on day 1. The patients received a total of 3 cycles, and each cycle took 2 weeks. If a toxic reaction of level 3 or 4 occurred, the chemotherapeutic dosage of the next cycle was reduced by 20%. Without clear surgical contraindications and with the patients’ informed consent, surgery was performed 3–4 weeks after the last chemotherapy treatment. During the treatment, if the patient presented with aggravated symptoms, experienced unbearable drug-specific toxicity or refused to continue chemotherapy due to persistent toxic reactions, the chemotherapy was stopped and surgery was conducted if distant metastases were not discovered.
All of the surgical operations in the enroled group were performed by a professional group of surgeons. The R0 resection surgery and D2 lymphadenectomy were required to be as far apart as possible. Postoperative chemotherapy was started 3–4 weeks after surgery, and the regimen was based on the results from the patients’ clinical and pathological evaluations. The patients with progressive disease (PD) or a histological response of 0–Ia according to the criteria described by the JCGC were given a modified DCF regimen. Otherwise, we continued the mFOLFOX6 or XELOX program. The pre and postoperative chemotherapies lasted half a year.
Assessment
Assessments were carried out before and after neoadjuvant chemotherapy, after surgery and after adjuvant chemotherapy. The following assessment techniques were applied: tumour marker analysis and abdominal CT, especially three-dimensional reconstruction CT, which was used as the major imaging assessment method before and after neoadjuvant chemotherapy. Additional imaging was undertaken depending on a clinical suspicion of recurrence. The tumour response to chemotherapy was assessed by an independent radiologist (WL), and the histological evaluation after preoperative therapy was assessed by an independent pathologist (DRZ), both using the criteria described by the JCGC.
For the specimens from radical surgery, the presence of tumour necrosis and fibrosis within the lesion was confirmed by the same experienced pathologist (DRZ), and the percentage of residual tumour cells, that is, graded histologic regression (GHR), within the lesion was recorded as 0–100%, with 0% representing no necrosis, or cellular or structural changes within the whole lesion and 100% representing an entire lesion that disappeared or was replaced by fibrous tissue without any viable tumour cells.
Toxicity was assessed according to the National Cancer Institute-Common Toxicity Criteria (NCI-CTC; version 3.0; Trotti et al, 2003).
Follow-up
Patients were followed-up at regular intervals, once every 3 months, either in a clinical visit or by telephone. The last follow-up date was 31 January 2015. During the out-patient review, tumour marker analysis and imaging examination, such as CT scans, were performed regularly (NCCN Clinical Practice Guidelines in Oncology, 2007).
Statistical analysis
The primary end points of this study were the response rate and R0 resection rate, and the secondary end points were the progression-free survival (PFS), OS and safety issues, including adverse events of chemotherapy and complications of surgery. Progression-free survival was defined as the period starting from the initial preoperative chemotherapy to the confirmation of progression of the disease by imaging or pathological diagnosis; OS was defined as the period from initial preoperative chemotherapy to the time of death for any reason. The full-analysis population included patients who had received at least one cycle of chemotherapy. The intention-to-treat population included those who completed the neoadjuvant chemotherapy and received radical surgery.
SAS statistical software 9.2 (SAS Institute Inc., Cary, NC, USA) was used for all of the statistical analyses. The T and N stages confirmed by CT were compared before and after neoadjuvant chemotherapy, and radiological downstaging was confirmed; the pathological downstaging was defined as a reduction in T stage or N stage of pathologic staging (ypTNM) compared with radiological staging before neoadjuvant chemotherapy. The correlation between clinicopathologic factors and the patients’ survival outcomes was analysed with univariate and multivariate analyses. A log-rank test was applied to compare the disjoint survival curves, and Wilcoxon’s test was used to compare the joint survival curves. The Cox-proportional hazards regression model was used to identify prognostic factors for survival. P<0.05 was statistically significant. All P-value results are from two-sided tests.
Results
General conditions
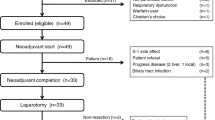
During the period from December 2006 to October 2012, there were 73 patients enroled in this study. Sixty patients were graded with an ECOG of 0 before chemotherapy (82.2%), and 13 patients were graded with an ECOG of 1 (17.8%). Seventy-three patients completed 211 cycles of neoadjuvant chemotherapy (1 cycle min. and 3 cycles max.), with a median of 3 cycles. Two patients stopped neoadjuvant chemotherapy and received surgery due to their Grade 3–4 nausea and vomiting after 1 cycle of chemotherapy, 1 patient elected to receive an operation due to PD after 2 cycles of neoadjuvant chemotherapy and 3 patients refused to continue chemotherapy and received surgery due to other reasons after 2 cycles of neoadjuvant chemotherapy. All of the patients’ basic information before chemotherapy is shown in Table 1. The procedure of this study is shown in Figure 1A.
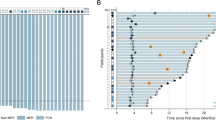
Prognostic value of the enroled patients.(A) Flow diagram from chemotherapy to surgery for 73 eligible patients. *Patients with PR, SD or a histological response of Ib-3 according to the JCGC criteria. ** Patients with PD or a histological response of 0–Ia according to JCGC criteria. (B) Distribution of graded histologic regression for patients who underwent radical surgery. (C) Progression-free survival. (D) Overall survival. (E) OS according to ypTNM stage for those who underwent radical surgery. (F) OS according to graded histologic response (⩾50%/<50%).
Among 73 patients, 67 (91.8%) received radical surgery, 4 (5.5%) received palliative surgery due to PD and 2 (2.7%) did not receive surgery due to PD. In patients who received radical surgery, 66 (90.4%) had a D2 lymphadenectomy and 1 (1.4%) received a D1 lymphadenectomy due to a large, chronic gastric ulcer perforation and severe adhesion of the surrounding organs.
Sixty-three (94.0%) patients received adjuvant chemotherapy for 354 cycles after radical surgery, with a median of 6 cycles (1 cycle min. and 9 cycles max.). Fifty-four (85.7%) patients received the mFOLFOX6 or XELOX regimen, and 9 (14.3%) received the modified DCF regimen. Table 2 depicts the pathologic characteristics of the resected tumours.
Efficacy
Prior to neoadjuvant chemotherapy, all the patients were assigned a clinical stage of either T stage- (T2, 12.3%; T3/4, 87.7%) or N stage- (N negative, 30.1%; N positive, 69.9%) based enhanced CT.
One (1.4%) patient did not receive a CT scan due to being lost to follow-up after chemotherapy. On the basis of the CT evaluations, T downstaging occurred in 17 (23.6%) of 72 patients, including 2 T2→T1, 2 T3→T1, 8 T3→T2 and 5 T4→T3, and N downstaging occurred in 12 (16.7%; N positive or N+→N negative or N−). Two groups were analysed for survival according to whether there was T or N downstaging, and this analysis showed that neither group was significantly different (P=0.236 for T, P=0.726 for N). According to the JCGC guidelines, there were no cases of CR, 33 cases (45.8%) of PR, 31 (43.0%) cases of SD and 8 (11.1%) cases of PD. Thus, the remission rate (CR+PR) was 45.8% and the disease control rate (CR+PR+SD) was 88.8%; the survival difference between the CR+PR group and the SD+PD group was not statistically significant (P=0.438).
Among 67 patients who had received radical surgery, 58 (86.6%) were identified as T3/4 by CT staging before chemotherapy and 45 (67.2%) were identified as N+, whereas only 48 (71.6%) were identified pathologically as T3/4 and 36 (53.7%) as N+ after surgery. The tumour stages according to neoadjuvant chemotherapy administration are illustrated in Table 3. Compared with pathological staging and CT staging before chemotherapy, T downstaging occurred in 22 patients (32.8%) and N downstaging occurred in 17 cases (25.4%). Although 32.8% of the patients displayed T downstaging after neoadjuvant chemotherapy, the difference did not reach a level of statistical significance between the downstaging and non-downstaging groups (P=0.324), whereas survival analysis showed a significant difference (P=0.017) between the N downstaging group and the non-downstaging group.
Figure 1B illustrates the distribution of GHR. All of the patients showed different levels of histological regression, in which 2 patients (3.0%) achieved a pathologically complete response (ypT0N0M0), 11 (16.4%) showed a response >90%, 20 cases (29.9%) showed a response ⩾2/3, and 33 cases (49.2%) showed a GHR ⩾50%.
Safety
During neoadjuvant chemotherapy, the most common toxicity was leucopoenia, mainly of Grade 1–2, with Grade 3 occurring in 9 (12.3%) cases, and Grade 4 occurring in 1 (1.4%) case. Grade 3 neutropenia occurred in 4 (5.5%) cases, and Grade 4 neutropenia occurred in 1 (1.4%) case; 13.6% of patients had thrombocytopenia, in which there were 2 (2.7%) cases of Grade 3 and 1 (1.4%) case of Grade 4. The non-haematological toxicity was mild, mainly of Grade 1 or Grade 2, and usually presented as nausea and vomiting, occurring in 49.3% of patients. One (1.4%) case suffered from Grade 3 vomiting, and 1 (1.4%) suffered from Grade 4 vomiting, and both of these patients stopped chemotherapy as a result and received surgery; 1 (1.4%) case suffered from Grade 3 diarrhoea. One (1.4%) case exhibited a Grade 3 ALT elevation and 2 (2.7%) cases suffered from drug fever. Grade 3 to 4 adverse reactions to chemotherapy are indicated in Table 4.
Among 71 patients who received surgery, 5 (7.0%) cases experienced operative complications. All of the complications were mild, and all of these patients received a D2 lymphadenectomy (Table 5) and recovered without any surgical intervention.
Survival analysis
Two (2.7%) of the 73 patients were lost to follow-up. The follow-up time ranged from 5.0 to 93.0 months, with a median of 37.0 months. Thirty-six (49.3%) cases showed disease progression, among which 33 (45.2%) died (as the disease progressed). The median PFS time was 56.0 months (Figure 1.C), and the median OS time was 76.0 months (Figure 1.D); the 1-year survival rate was 84.9%, the 2-year survival rate was 63.0% and the 3-year survival rate was 61.5%.
Univariate analysis was performed on 67 patients who received radical surgery regarding the clinicopathologic factors that might influence survival. The results demonstrated that factors such as local invasion, Lauren classification, N staging before chemotherapy and ypTNM staging (Figure 1.E) were correlated with OS. We also examined the association between OS and GHR thresholds of 90%, 66.7%, 60%, 50%, 45%, 40% and 35%, from which it was found that when the thresholds were 50% (Figure 1F) or 66.7%%, the GHR of the primary lesion was correlated with survival.
Multivariate analysis was performed by incorporating the factors of sex, age, tumour primary site, Lauren classification, local invasions, pathologic T and N downstaging, GHR of the primary lesion (⩾50%/<50% or ⩾2/3/<2/3), TNM staging before chemotherapy and ypTNM with COX regression, and the results demonstrated that only Lauren classification and ypTNM had a statistically significant association with OS (details in Table 6).
Discussion
The treatment of gastric cancer, especially advanced gastric cancer, has always been a challenge for surgeons, oncologists and radiologists. Because there is no standard neoadjuvant chemotherapy regimen, the FOLFOX regimen has been widely used in treating advanced gastric cancer and was adopted in this study. Since 2001, the FOLFOX regimen has become one of the most common treatments for advanced gastric cancer (Louvet et al, 2002; Kim et al, 2003; AI-Batran et al, 2004; Chao et al, 2004; De Vita et al, 2005; Hwang et al, 2008; Keam et al, 2008). To date, several clinical studies have demonstrated that the FOLFOX regimen, as a neoadjuvant chemotherapy regimen, can improve the effectiveness of locally advanced gastric cancer, with response rates of 50% to 69.7% (Li et al, 2012; Zhang et al, 2012).
In the current study, one of the primary end points was the response rate. The response rate is mainly evaluated based on radiologic or pathologic criteria. According to rigorous imaging evaluation, no CR cases were found, but 45.8% of patients reached PR after three-cycle chemotherapy on average, with a response rate of 45.8% in this study. Thus, our mFOLFOX6 regimen showed similar efficacy to the reported FOLFOX regimen (Zhang et al, 2012). Unfortunately, the survival analysis performed by grouping patients into CR+PR or SD+PD was not significantly different, indicating that the impact of the effectiveness of neoadjuvant chemotherapy on prognosis cannot be evaluated by CT imaging only, which was consistent with the conclusion reported by Kurokawa et al (2014).
Histological response is another important assessment method. The patients who received radical surgery all showed different levels of GHR, with a 3.0% complete regression rate, which was consistent with the 0–15% rate reported by previous prospective studies (Ott et al, 2003; Cunningham et al, 2006; Jary et al, 2014; Wang et al, 2014; Yoshikawa et al, 2014); the GHR of 49.2% cases in this study was over 50%, which was higher than 39% reported in the literature (Ferri et al, 2012), in which 84% were lower oesophageal or gastroesophageal junction cancers, whereas only 13.6% cases in this study were proximal gastric cancer. Gastric cancer in different sites might react differently towards chemotherapy. Two patients who had pCR only had partial clinical responses, showing that CT imaging results did not always agree with histological findings.
According to the survival analysis, postoperative pathological factors seemed to be better surrogate end points for OS in studies of neoadjuvant therapy for gastric cancer. Although multivariate analysis did not demonstrate that a GHR ⩾50%/<50% was an independent prognostic factor, univariate analysis revealed that a GHR of 50% between two groups reached statistical significance. It was a practical issue to clarify the GHR threshold that indicated whether neoadjuvant chemotherapy was effective. According to the widely used criteria described by Becker et al (2003) and the JCGC, the response rate of cases was lowered, and recent literature also reported that there was no difference in survival rates between the responder and non-responder groups on multivariate analysis (Fujitani et al, 2012; Schmidt et al, 2014). When taking 50% as the cut-off value, the 3-year survival rate of patients with a GHR >50% was significantly higher than those with a GHR <50% (69% vs 44%, P=0.01; Mansour et al, 2007). In the current study, the groups with a GHR ⩾2/3 vs <2/3 and ⩾50% vs <50% all showed a correlation with survival, but the ratio of GHR ⩾2/3 was only 29.9% (the ratio of GHR⩾50% could be seen in our paper). Hence, a GHR ⩾50%/<50% could be applied as the major evaluation criterion for gastric cancer to help formulate a postoperative adjuvant chemotherapy regimen. However, further investigation is necessary to confirm the conclusion that the GHR threshold of 50% can lead to significant survival benefits.
In this study, both univariate analysis and multivariate analysis showed that postoperative TNM was the most significant independent prognostic factor, whereas the clinical stage before neoadjuvant chemotherapy was not as reliable as a surrogate marker for OS, which was consistent with the conclusions reported by Schmidt et al (2014) and Davies et al (2014). Thus, we could infer that patients with a GHR over 50% after neoadjuvant chemotherapy and whose primary tumours were downstaged to a lower TNM stage could be the real beneficiaries and exhibit a longer OS.
Of all of the prognostic factors in the univariate analysis, only the Lauren classification maintained statistical significance on multivariate analysis. Due to contradictions with other studies’ results (Fujitani et al, 2012; Schmidt et al, 2014), this issue is worth further investigation.
The R0 resection rate was another end point of this study. Of 73 patients, 91.8% received radical surgery, which was considerably higher than the 69.3% reported by the MAGIC trial using ECF as a neoadjuvant chemotherapy regimen (Cunningham et al, 2006); the FFCD 9703 trial published in 2011 (Ychou et al, 2011) also showed the efficacy of cisplatin+5-FU in gastric cancer, but only 84% of patients received radical surgery. Our study showed similar efficacy compared with other clinical trials using FOLFOX as a neoadjuvant chemotherapy regimen, citing a rate between 86% and 92.1% (Li et al, 2012; Zhang et al, 2012). Thus, patients with gastric cancer could have a higher R0 resection rate when using FOLFOX as a neoadjuvant chemotherapy regimen.
The current study confirmed the high tolerability of the regimen. Similar to other studies in gastric cancer with the FOLFOX regimen (AI-Batran et al, 2004; De Vita et al, 2005; Li et al, 2012; Zhang et al, 2012), most toxicities were Grade 1 or 2, and only 21.8% of the patients experienced Grade 3 or 4 toxicity, without chemotherapy-associated death or serious complications. Of the 67 patients who received radical surgery, 63 (94.0%; accounting for 86.3% of the total inclusive population) continued postoperative chemotherapy, which was slightly higher than the 54.8% in the MAGIC trial (Cunningham et al, 2006) and 47.8% in the FFCD 9703 trial (Ychou et al, 2011).
D2 gastrectomy has been recommended as a standard practice due to its efficacy and safety, but the safety of D2 resection after chemotherapy has rarely been evaluated, though it is of particular concern for surgeons. Although the MAGIC trial (Cunningham et al, 2006) and a retrospective analysis from China (Li et al, 2011) have demonstrated that there was no difference in perioperative morbidity with and without neoadjuvant chemotherapy, the FFCD 9703 trial (Ychou et al, 2011), 40954 trial (Schuhmacher et al, 2010) and other non-randomised studies (Fujitani et al, 2007; An et al, 2012) demonstrated a trend towards a higher, although non-significant, postoperative morbidity rate in patients who received neoadjuvant chemotherapy. However, the limitation of these trials has been the non-uniform performance of D2 gastrectomy. Due to the conflicting reports, Shrikhande et al (2013) prospectively analysed 139 cases who received neoadjuvant chemotherapy, in which 126 cases received a D2 gastrectomy. The morbidity in the latter was 12% and the mortality was zero, whereas the morbidity in the control group of those who received upfront surgery was 22.6% without death. Another phase II study with XELOX as a neoadjuvant chemotherapy regimen also showed a low morbidity. In the present study, 66 cases received a D2 gastrectomy after neoadjuvant chemotherapy, and only 5 cases suffered from complications with an occurrence rate of 7.6% and no surgery-associated death. D2 gastrectomy after neoadjuvant chemotherapy was safe and effective.
In conclusion, mFOLFOX6 is a safe, effective and well-tolerated regimen of neoadjuvant chemotherapy for the treatment of locally advanced gastric cancer. The postoperative TNM stage was the most significant independent prognostic factor. We suggest that a GHR ⩾50%/<50% be used as the criterion for evaluating the curative effects of neoadjuvant chemotherapy to guide the selection of postoperative adjuvant chemotherapy regimens. Patients with a GHR ⩾50% may consider continuing their use of the original method of postoperative chemotherapy. However, this was a single-institution study with a small number of cases and without control groups; thus, an appropriately powered randomised trial is necessary before any firm conclusion can be established.
Change history
14 June 2016
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
AI-Batran SE, Atmaca A, Hegewisch-Becker S, Jaeqer D, Hahnfeld S, Rummel MJ, Seipelt G, Rost A, Orth J, Knuth A, Jaeger E (2004) Phase II trial of biweekly infusional fluorouracil, folinic acid, and oxaliplatin in patients with advanced gastric cancer. J Clin Oncol 22: 658–663.
An JY, Kim KM, Kim YM, Cheong JH, Hyung WJ, Noh SH (2012) Surgical complications in gastric cancer patients preoperatively treated with chemotherapy: their risk factors and clinical relevance. Ann Surg Oncol 19: 2452–2458.
Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, Bottcher K, Siewert JR, Hofler H (2003) Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 98: 1521–1530.
Berger AC, Farma J, Scott WJ, Freedman G, Weiner L, Cheng JD, Wang H, Goldberg M (2005) Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol 23: 4330–4337.
Brenner B, Shah MA, Karpeh MS, Gonen M, Brennan MF, Coit DG, Klimstra DS, Tang LH, Kelsen DP (2006) A phase II trial of neoadjuvant cisplatin-fluorouracil followed by postoperative intraperitoneal floxuridine-leucovorin in patients with locallyadvanced gastric cancer. Ann Oncol 17: 1404–1411.
Breslin TM, Hess KR, Harbison DB, Jean ME, Cleary KR, Dacklw AP, Wolff RA, Abbruzzese JL, Janjan NA, Crane CH, Vauthey JN, Lee JE, Pisters PW, Evans DB (2001) Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol 8: 123–132.
Chao Y, Yeh KH, Chang CJ, Chen LT, Chao TY, Wu MF, Chang CS, Chang JY, Chung CY, Kao WY, Hsieh RK, Cheng AL (2004) Phase II study of weekly oxaliplatin and 24-h infusion of high-dose 5-fluorouracil and folinic acid in the treatment of advanced gastric cancer. Br J Cancer 91: 453–458.
Chirieac LR, Swisher SG, Ajani JA, Komaki RR, Correa AM, Morris JS, Roth JA, Rashid A, Hamilton SR, Wu TT (2005) Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer 103: 1347–1355.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, Participants MT (2006) Perioperrative chemotherapy versus surgery alone for respectable gastroesophageal cancer. N Engl J Med 355: 11–20.
Davies AR, Gossage JA, Zylstra J, Mattsson F, Lagergren J, Maisey N, Smyth EC, Cunningham D, Allum WH, Mason RC (2014) Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol 32: 2983–2990.
De Vita F, Orditura M, Matano E, Bianco R, Carlomagno C, Infusino S, Damiano V, Simeone E, Diadema MR, Lieto E, Castellano P, Pepe S, De Placido S, Galizia G, Di Martino N, Ciardiello F, Catalano G, Bianco AR (2005) A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer 92: 1644–1649.
Ferri LE, Ades S, Alcindor T, Chasen M, Marcus V, Hickeson M, Artho G, Thirlwell MP (2012) Perioperative docetaxel, cisplatin, and 5-fluorouracil (DCF) for locally advanced esophageal and gastric adenocarcinoma: a multicenter phase II trial. Ann Oncol 23: 1512–1517.
Fujitani K, Ajani JA, Crane CH, Feig BW, Pisters PW, Janjan N, Walsh GL, Swisher SG, Vaporciyan AA, Rice D, Welch A, Baker J, Faust J, Mansfield PF (2007) Impact of induction chemotherapy and preoperative chemoradiotherapy on operative morbidity and mortality in patients with locoregional adenocarcinoma of the stomach or gastroesophageal junction. Ann Surg Oncol 14: 2010–2017.
Fujitani K, Mano M, Hirao M, Kodama Y, Tsujinaka T (2012) Posttherapy nodal status, not graded histologic response, predicts survival after neoadjuvant chemotherapy for advanced gastric cancer. Ann Surg Oncol 19: 1936–1943.
Gaca JG, Petersen RP, Peterson BL, Harpole DH Jr, D'Amico TA, Pappas TN, Seigler HF, Wolfe WG, Tyler DS (2006) Pathologic nodal status predicts disease-free survival after neoadjuvant chemoradiation for gastroesophageal junction carcinoma. Ann Surg Oncol 13: 340–346.
Gu Y, Swisher SG, Ajani JA, Correa AM, Hofstetter WL, Liao Z, Komaki RR, Rashid A, Hamilton SR, Wu TT (2006) The number of lymph nodes with metastasis predicts survival in patients with esophageal or esophagogastric junction adenocarcinoma who receive preoperative chemoradiation. Cancer 106: 1017–1025.
Husband JE, Schwartz LH, Spencer J, Ollivier L, King DM, Johnson R, Reznek R International Cancer Imaging Society (2004) Evaluation of the response to treatment of solid tumours-a consensus statement of the International Cancer Imaging Society. Br J Cancer 90: 2256–2260.
Hwang WS, Chao TY, Lin SF, Chung CY, Chiu CF, Chang YF, Chen PM, Chiou TJ (2008) Phase II study of oxaliplatin in combination with continuous infusion of 5-fluorouracil/leucovorin as first-line chemotherapy in patients with advanced gastric cancer. Anticancer Drugs 19: 283–288.
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14: 101–102.
Jary M, Ghiringhelli F, Jacquin M, Fein F, Nguyen T, Cleau D, Nerich V, EI Gani M, Mathieu P, Valmary-Degano S, Arnould L, Lassabe C, Lamfichekh N, Fratte S, Paget-Bailly S, Bonnetain F, Borg C, Kim S (2014) Phase II multicentre study of efficacy and feasibility of dose-intensified preoperative weekly cisplatin, epirubicin, and paclitaxel (PET) in resectable gastroesophageal cancer. Cancer Chemother Pharmacol 74: 141–150.
Keam B, Im SA, Han SW, Ham HS, Kim MA, Oh DY, Lee SH, Kim JH, Kim DW, Kim TY, Heo DS, Kim WH, Bang YJ (2008) Modified FOLFOX-6 chemotherapy in advanced gastric cancer: results of phase II study and comprehensive analysis of polymorphisms as a predictive and prognostic marker. BMC Cancer 8: 148.
Kim DY, Kim JH, Lee SH, Kim TY, Heo DS, Bang YJ, Kim NK (2003) Phase II study of oxaplatin, 5-fluorouracil and leucovorin in previously platinum-treated patients with advanced gastric cancer. Ann Oncol 14: 383–387.
Kurokawa Y, Shibata T, Sasako M, Sano T, Tsuburaya A, Iwasaki Y, Fukuda H (2014) Validity of response assessment criteria in neoadjuvant chemotherapy for gastric cancer (JCOG0507-A). Gastric Cancer 17: 514–521.
Li W, Qin J, Sun YH, Liu TS . Neoadjuvant chemotherapy for advanced gastric cancer: a meta-analysis (2010) World J Gastroenterol 16: 5621–5628.
Li ZY, Koh CE, Bu ZD, Wu AW, Zhang LH, Wu XJ, Wu Q, Zong XL, Ren H, Tang L, Zhang XP, Li JY, Hu Y, Shen L, Ji JF (2012) Neoadjuvant chemotherapy with FOLFOX: improved outcomes in Chinese patients with locally advanced gastric cancer. J Surg Oncol 105: 793–799.
Li ZY, Shan F, Zhang LH, Bu ZD, Wu AW, Wu XJ, Zong XL, Wu Q, Ren H, Ji JF (2011) Complications after radical gastrectomy following FOLFOX7 neoadjuvant chemotherapy for gastric cancer. World J Surg Oncol 9: 110.
Louvet C, Andre T, Tigaud JM, Gamelin E, Douillard JY, Brunet R, Francois E, Jacob JH, Levoir D, Taamma A, Rougier P, Cvitkovic E, de Gramont A (2002) Phase II study of oxaliplatin, fluorouracil, and folinic acid in locally advanced or metastatic gastric cancer patients. J Clin Oncol 20: 4543–4548.
Lowy AM, Mansfiled PF, Leach SD, Pazdur R, Dumas P, Ajani JA (1999) Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg 229: 303–308.
Lutz MP, Zalcberg JR, Ducreux M, Ajani JA, Allum W, Aust D, Bang YJ, Cascinu S, Holscher A, Jankowski J, Jansen EP, Kisslich R, Lordick F, Mariette C, Moehler M, Oyama T, Roth A, Rueschoff J, Ruhstaller T, Seruca R, Stahl M, Sterzing F, van Cutsem E, van der Gaast A, van Lanschot J, Ychou M, Otto F First St Gallen EORTC Gastrointestinal Cancer Conference 2012 Expert Panel (2012) Highlights of the EORTC St. Gallen International Expert Consensus on the primary therapy of gastric, gastroesophageal and oesophageal cancer-differential treatment strategies for subtypes of early gastroesophageal cancer. Eur J Cancer 48: 2941–2953.
Mansour JC, Tang L, Shah M, Bentrem D, Klimstra DS, Gonen M, Kelsen DP, Brennan MF, Coit DG (2007) Does graded histologic response after neoadjuvant chemotherapy predict survival for completely resected gastric cancer? Ann Surg Oncol 14: 3412–3418.
NCCN Clinical Practice Guidelines in Oncology (2007) gastric cancer V.1:10.
Onaitis MW, Noone RB, Fields R, Hurwitz H, Morse M, Jowell P, McGrath K, Lee C, Anscher MS, Clary B, Mantyh C, Pappas TN, Ludwiq K, Seiqler HF, Tyler DS (2001) Complete response to neoadjuvantchemoradiation for rectal cancer does not influence survival. Ann Surg Oncol 8: 801–806.
Ott K, Fink U, Becker K, Stahl A, Dittler HJ, Busch R, Stein H, Lordick F, Link T, Schwaiger M, Siewert JR, Weber WA (2003) Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol 21: 4604–4610.
Ruo L, Tickoo S, Klimstra D, Minsky BD, Saltz L, Mazumdar M, Paty PB, Wong WD, Larson SM, Cohen AM, Guillem JG (2002) Long-term prognostic significance of extent of rectal cancer response to preoperative radiation and chemotherapy. Ann Surg 236: 75–81.
Schmidt T, Sicic L, Blank S, Becker K, Weichert W, Bruckner T, Parakonthun T, Langer R, Buchler MW, Siewert JR, Lordick F, Ott K (2014) Prognostic value of histopathological regression in 850 neoadjuvantly treated oesophagogastric adenocarcinomas. Br J Cancer 110: 1712–1720.
Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J, Ott K, Hoelscher A, Schneider PM, Bechstein W, Wilke H, Lutz MP, Nordlinger B, Van Cutsem E, Siewert JR, Schlag PM (2010) Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol 28: 5210–5218.
Shrikhande SV, Barreto SG, Talole SD, Vinchurkar K, Annaiah S, Suradkar K, Mehta S, Goel M (2013) D2 lymphadenectomy is not only safe but necessary in the era of neoadjuvant chemotherapy. World J Surg Oncol 11: 31.
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse effects ofcancer treatment. Semin Radiat Oncol 13: 176–181.
Wang Y, Yu YY, Li W, Feng Y, Hou J, Ji Y, Sun YH, Shen KT, Shen ZB, Qin XY, Liu TS (2014) A phase II trial of Xeloda and oxaliplatin (XELOX) neo-adjuvant chemotherapy followed by surgery for advanced gastric cancer patients with para-aortic lymph node metastasis. Cancer Chemother Pharmacol 73: 1155–1161.
Wilke H, Preusser P, Fink U, Gunzer U, Meyer HJ, Meyer J, Siewert JR, Achterrath W, Lenaz L, Knipp H (1989) Preoperative chemotherapy in locally advanced and nonresectable gastric cancer: a phase II study with etoposide, doxorubicin, and cisplatin. J Clin Oncol 7: 1318–1326.
Ychou M, Boige V, Pignon JP, Conroy T, Bouche O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Genève J, Lasser P, Rougier P (2011) Perioperative chemotherapy compared with surgery alone for resectable gastro- esophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 29 (13): 1715–1721.
Yoshikawa T, Tanabe K, Nishikawa K, Ito Y, Matsui T, Kimura Y, Hasegawa S, Aoyama T, Hayashi T, Morita S, Miyashita Y, Tsuburaya A, Sakamoto J (2014) Accuracy of CT staging of locally advanced gastric cancer after neoadjuvant chemotherapy: cohort evaluation within a randomized phase II study. Ann Surg Oncol 21: 385–389.
Zhang J, Chen RX, Zhang J, Cai J, Meng H, Wu GC, Zhang ZT, Wang Y, Wang KL (2012) Efficacy and safety of neoadjuvant chemotherapy with modified FOLFOX7 regimen on the treatment of advanced gastric cancer. Chin Med J 125: 2144–2150.
Acknowledgements
We thank all of the patients who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Wang, X., Zhao, L., Liu, H. et al. A phase II study of a modified FOLFOX6 regimen as neoadjuvant chemotherapy for locally advanced gastric cancer. Br J Cancer 114, 1326–1333 (2016). https://doi.org/10.1038/bjc.2016.126
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2016.126
Keywords
This article is cited by
-
Oncological risk of proximal gastrectomy for proximal advanced gastric cancer after neoadjuvant chemotherapy
BMC Cancer (2024)
-
Neoadjuvant chemotherapy in locally advanced colon cancer: a systematic review and meta-analysis
International Journal of Colorectal Disease (2021)
-
The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer
Cancer Communications (2019)
-
Pathological evaluation of neoadjuvant chemotherapy in advanced gastric cancer
World Journal of Surgical Oncology (2019)
-
Outcomes of preoperative S-1 and docetaxel combination chemotherapy in patients with locally advanced gastric cancer
Cancer Chemotherapy and Pharmacology (2019)