Abstract
Background:
To evaluate the possible association between paediatric head computed tomography (CT) examination and increased subsequent risk of malignancy and benign brain tumour.
Methods:
In the exposed cohort, 24 418 participants under 18 years of age, who underwent head CT examination between 1998 and 2006, were identified from the Taiwan National Health Insurance Research Database (NHIRD). Patients were followed up until a diagnosis of malignant disease or benign brain tumour, withdrawal from the National Health Insurance (NHI) system, or at the end of 2008.
Results:
The overall risk was not significantly different in the two cohorts (incidence rate=36.72 per 100 000 person-years in the exposed cohort, 28.48 per 100 000 person-years in the unexposed cohort, hazard ratio (HR)=1.29, 95% confidence interval (CI)=0.90–1.85). The risk of benign brain tumour was significantly higher in the exposed cohort than in the unexposed cohort (HR=2.97, 95% CI=1.49–5.93). The frequency of CT examination showed strong correlation with the subsequent overall risk of malignancy and benign brain tumour.
Conclusions:
We found that paediatric head CT examination was associated with an increased incidence of benign brain tumour. A large-scale study with longer follow-up is necessary to confirm this result.
Similar content being viewed by others
Main
Previous studies on survivors of the Hiroshima and Nagasaki atomic bombs, as well as cancer patients who developed secondary cancer within the radiation field, have shown that exposure to high-dose radiation increases the risk of leukaemia and various solid tumours (Preston et al, 2007; Bednarz et al, 2010; Newhauser and Durante, 2011). Radiation exposure in paediatric populations is of particular concern because of their increased sensitivity to radiation and higher number of years in which carcinogenesis might occur.
According to the National Council on Radiation Protection and Measurements (NCRP) report no. 160, medical radiation exposure to the US citizen has increased three-fold within the past two decades, providing 48% of the total ionising radiation source. Computed tomography (CT) scanning provided the most substantial contribution to this value (National Council on Radiation Protection and Measurements, 2009). Over the past three decades, the rate of CT use has shown marked increases (∼20-fold) (Brenner and Hall, 2007; Mettler et al, 2009), and this trend is likely to continue as its clinical application further increases rapidly (Fazel et al, 2009). Investigators have observed the largest increases in CT use within the categories of paediatric diagnosis (National Council on Radiation Protection and Measurements, Scientific Commitee 6-2 on Radiation Exposure of the US. Population, 2009). Despite the clinical value of CT, concerns exist about the potential cancer risk resulting from the ionising radiation associated with its use, particularly in the paediatric population.
In Taiwan, the Taiwan National Health Insurance Research Database (NHIRD), one of the largest administrative health-care databases worldwide, contains de-identified medical claims from 99% of Taiwan’s 23.74 million people. The National Health Research Institute (NHRI) compiled the NHIRD using medical claims in the National Health Insurance (NHI) program, a single-payer universal health insurance program initiated in 1995, with more than 99% of the island’s population enrolled in the scheme. The NHIRD is available for research purposes in Taiwan, and epidemiologic studies have extensively used its data (Chen et al, 2011; Huang et al, 2012; Liang et al, 2012; Kao et al, 2013). Cheng et al (2011) validated the accuracy of the diagnoses of diseases and drug prescriptions in the NHIRD. These data enabled our evaluation of the possible increased subsequent risk of malignancy and benign brain tumour resulting from head CT scan in a paediatric population using a nation-wide population-based cohort study.
Materials and Methods
Data source
We obtained data from the database that included 50% insured children who were randomly selected from all insured children in Taiwan. Children claims data from the NHIRD from January 1996 to December 2008, including all medical diagnoses (included inpatient and outpatient), procedures, and patient details, were used in this study. The catastrophic illness certificate database (CICD) was also used to identify cancer patients (all cancer patients were histologically or cytologically confirmed before catastrophic illness certification). The details of the claims files are described in the NHIRD website. Diagnoses in the database were coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code.
Study cohorts
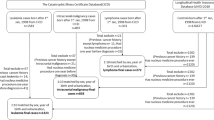
Children (under 18 years of age) who underwent head CT examination from 1998 to 2006 were selected using the ICD-9-CM procedure code 87.03 (n=28 185) as the exposed cohort, and children with disorders who may have an increased cancer risk (neurofibromatosis (ICD-9-CM 237.7), hamartomas (ICD-9-CM 759.6), multiple endocrine neoplasia (ICD-9-CM 258.01-258.03), and disorders of adrenal gland (ICD-9-CM255)), any cancer history, or cancer development within the first 2 years follow-up were excluded for minimising the selection bias and the screening effect (n=3767). The date for head CT examination was defined as the index date and the start of follow-up set at 2 years after the index date (lag period of 2 years). For each case identified, identical exclusion criteria were used to randomly select an unexposed cohort in a 1 : 4 ratio. The unexposed cohort was matched with the exposed patients for sex, age, year of index, and month of index. Those who received head CT before the end point were excluded. Figure 1 displays the flowchart for the selection of participants. All enrolled study subjects were followed up until the diagnosis of malignant disease (ICD-9-CM 140-208), benign brain tumour (ICD-9-CM 225, 227.3, 227.4), death, withdrawal from the NHI system, or at the end of 2008.
Statistical analysis
Baseline characteristics of the exposed and unexposed cohorts were compared using the χ2-test. The incidence densities of the two cohorts were calculated according to age and sex. Multivariate Cox’s proportion hazard regression was used to examine the effects of CT examination on the risk of cancer compared with the unexposed cohort, as shown using hazard ratios (HR) with 95% confidence intervals (CI). A two-tailed P-value of <0.05 was considered statistically significant. All statistical analyses were performed using the SAS statistical software (version 9.2 for Windows; SAS Institute, Inc., Cary, NC, USA).
Results
In the exposed and unexposed cohort, the peak age was 0–6 years (40.0%), followed by 13–18 years (38.8%) and 7–12 years (21.2%). Boys received head CT examination more frequently than girls (60.9% vs 39.1%). In the exposed cohort, most children underwent head CT examination once during the study period (93.4%), followed by twice and more than twice (5.42% and 1.20%, respectively).
In the cohorts, 5.04% of the participants were lost to follow-up at the end of 2008. The most common reason of attrition from the cohorts was to change the participant’s title or change their insurance branches (4.00%). The second most common reason was to drop out of the insurance owing to death, being missing, or foreigners who lose their qualification for insurance (0.56%). Other causes included emigration, custody, and unknown cause (0.48%).
The overall risk of malignancy and benign brain tumour was not significantly different in the two cohorts (incidence rate=36.72 per 100 000 person-years in the CT cohort, 28.48 per 100 000 person-years in the non-CT cohort, HR=1.29, 95% CI=0.90–1.85; Table 1). The risks of all brain tumours were significantly higher in the exposed cohort than in the unexposed cohort (HR=2.56, 95% CI=1.44–4.54), especially in the participants aged 0–6 years at exposure (HR=3.16, 95% CI=1.18–8.49; Table 2). Children who underwent CT examination also had a higher risk for benign brain tumour (HR=2.97, 95% CI=1.49–5.93; Table 1) than unexposed children, and only those aged 7–12 years old at exposure had a higher risk (HR=6.20, 95% CI=1.01–36.0; Table 2). If stratified according to sex, the cohort comprising girls who were exposed had a 2.48- and 3.15-fold higher relative risk of developing all brain tumours and benign brain tumours than the cohort comprising girls who were unexposed (95% CI=1.03–5.99 and 1.17–8.45; Table 2). Similarly, the cohort comprising boys who were exposed had a 2.62- and 2.82-fold higher relative risk of developing all brain tumours and benign brain tumours than the cohort comprising boys who were unexposed (95% CI=1.23–5.59 and 1.08–7.42).
As shown in Table 3, the overall malignancy and all brain tumour risks were higher within 4–5 years since the first exposure compared with the unexposed cohort (HR=1.77 and 3.62, 95% CI=1.07–2.92 and 1.47–8.91, respectively). The risk of benign brain tumour was the highest within the third year since the first exposure (HR=5.34, 95% CI=1.20–23.9), followed by 4–5 years (HR=3.45, 95% CI=1.16–10.3) and >5 years (HR=1.81, 95% CI=0.56–5.87) in the exposure cohort compared with the unexposed cohort. The risk of all brain tumours increased with more frequent CT examination (increase in HR from 2.32 to 10.4 with the increase in frequency from 1 to ⩾3, trend test P=0.0001) compared with the unexposed cohort (Table 4).
Discussion
To the best of our knowledge, this is the first cohort study to investigate the association between paediatric head CT and subsequent risk of malignancy and benign brain tumour outside of the UK and Australia. Using our nation-wide database, we identified that paediatric head CT scanning is associated significantly with the subsequent risk of benign brain tumour, observing a 2.97-fold higher risk in the exposed cohort compared with the unexposed cohort.
In this study, the risk of benign brain tumour varied with age at exposure. Participants aged 7–12 years were more susceptible to benign brain tumour. For the overall brain tumours, those aged 0–6 years had the highest risk. In a previous report on the survivors of atomic-bomb exposure and the Chernobyl accident, the younger population had a greater radiation cancer risk (United Nations Scientific Committee on the Effects of Atomic Radiation, 2010; Ivanov et al, 2012). In contrast, an epidemiological study on cancer risk following CT examination observed a higher risk of brain tumour with increasing age at exposure during childhood and adolescence (Pearce et al, 2012). However, the authors did not observe a similar trend for the risk of leukaemia. Therefore, heterogeneity in the association between the risk of radiation-induced neoplasm and age at exposure exists across studies, and it is not possible to draw firm conclusions on how these risks vary according to age at exposure.
Our study results showed that the frequency of CT scan correlated significantly with the subsequent risk of benign brain tumour. This observation further supports our finding that childhood exposure to CT scans increases the future risk of benign brain tumour in Taiwan. With exclusion interval of 2 years, the relative risk of benign brain tumour was significantly higher in the exposed cohort than in the controls. This might imply that our results were less likely from the bias caused by a screening effect. If we did not set the exclusion period, a small fraction of participants in the exposed cohort might be diagnosed to have cancer or brain tumours soon after the first CT scan. Obviously, they were not radiation-related events owing to the short latent period. This would bias our result to survey the effect of CT-related risk.
Previous studies estimated the risk of radiation-induced cancer resulting from paediatric CT using data from Japanese atomic-bomb survivors (Brenner et al, 2001; Preston et al, 2007). However, radiation effect from CT scans may be different from that of atomic-bomb exposure. In 2012, Pearce et al (2012) published the first epidemiological study to estimate the risk of brain tumour and leukaemia resulting from paediatric CT examinations. It was a large retrospective cohort study conducted in the United Kingdom. The authors collected data from ∼280 000 CT scans from patients aged younger than 22 years between 1985 and 2002 from the radiology information system database of the National Health Service (NHS) system. They linked to the NHS Central Registry to obtain information on subsequent cancer diagnoses, and identified a small, but significant, increased risk of leukaemia (excess relative risk per m Gy, 0.036) and brain tumour (excess relative risk per m Gy, 0.023). They estimated that in children aged 10 years and younger, within 10 years of the first CT scan, one excess case of brain tumour and one excess case of leukaemia per 10 000 head CT scans occur. Their study was the first to use an epidemiological survey to evaluate the risk of cancer resulting from CT scans in childhood and adolescence. In their study, they reported the result of combined malignant and benign brain tumour. In 2013, Mathews et al published their data linkage study of cancer risk in 680 000 people aged 0–19 years exposed to CT scans from Australian Medicare records (Mathews et al, 2013). On the basis of a 1-year lag period, incidence of all types of cancers combined was 24% greater for exposed than for unexposed people. Among them, malignant brain cancer had the highest relative risk (incidence rate ratio (IRR)=2.13, 95% CI=1.88–2.41). This may imply that brain tissue is the one associated with high radiosensitivity. They included all CT scans, not just brain CT scans. Unlike our study, they do not survey the risk of benign brain tumour. We reported the results of malignant brain tumour, benign brain tumour, and combined malignant and benign brain tumour.
The study by Pearce et al (2012) assessed the risk of leukaemia and brain tumour associated with a uniform estimated absorbed dose of radiation in the red bone marrow and brain tissue from all CT scans. In our study, however, we did not use a uniform estimated specific organ-absorbed dose to evaluate the dose–response relationship because the actual absorbed doses from CT scans can differ considerably according to age, sex, equipment type, shape and size of the head, the use of contrast, protocols of scanning, purpose of examinations, and so on. These can result in large variations in effective radiation doses. Smith-Bindman et al (2009) estimated the effective radiation doses for the 11 most frequent CT study types in 1 119 consecutive adult patients at four institutions in San Francisco Bay in 2008 and identified marked variations. In any routine head CT scan, the effective dose varied significantly within and across institutions, with a range of 0.3–6 mSv. The median effective dose ranged from 2 m Sv for a routine head CT (interquartile range, 2–3 m Sv) to 14 m Sv (interquartile range, 9–20 m Sv) for a suspected stroke CT. The variations displayed no discernable pattern and occurred within and across institutions. Therefore, using a uniform estimated dose for all CT scans to calculate the actual radiation exposure is unlikely to provide accurate values.
The exclusion interval from exposure to cancer diagnosis was set at 2 years in the present study. So far, there is uncertainty regarding how long the latent phase between the radiation exposure and development of associated cancers is. The data of radiation-induced cancer mainly arose from survivors of the atomic bomb and patients who received radiation therapy. They were found to have a greater risk of subsequent leukaemia and solid tumours. Subsequent tumours may occur several years after radiation exposure. Nevertheless, previous studies have reported a greater risk of secondary solid tumours and leukaemia even with a short follow-up. The excess risk started to appear during 1–3 years after the exposure and reached the peak at 6–7 years or at the age at which the cancer was normally prone to develop. For example, Swerdlow et al (1992) conducted a cohort study of 2846 patients who were treated for their Hodgkin’s disease during 1970–1987. They found a 2.4-fold increased risk of lung cancer during 0–4 years of follow-up. Life span study (LSS) is a cohort study to evaluate the effects of radiation exposure in the Japanese atomic-bomb survivors, which consisted of 93 000 atomic-bomb survivors and 27 000 controls. It showed that the excess absolute rates in solid cancers appeared to increase throughout the study period, providing further evidence that radiation-associated increases in cancer rates persist throughout life regardless of age at exposure (Preston et al, 2007). Other studies also showed that the excess leukaemia cases could occur as early as 1–3 years after exposure (Boice et al, 1987; Curtis et al, 1992; Inskip et al, 1993; Curtis et al, 1994; Weiss et al, 1994, 1995). The median interval between radiation exposure and occurrence of secondary cancers may be several decades, but the excess risk started to appear as early as 1–3 years. Therefore, we set the exclusion interval at 2 years.
An unexpected observation in this study should be interpreted with caution. We found that there is a trend of decreased risk of benign brain tumour with longer interval from exposure (Table 3, 5.34 for 3 years after exposure, 3.45 for the period of 4–5 years, and 1.81 for the period of >5 years). The cancer risk during these intervals should be increased with the duration of follow-up rather than decreased. We could not fully understand the true cause for this issue. This phenomenon was also observed in the previous studies, which showed that the occurrence of radiation-associated neoplasms consisted of an early pulse of increased risk followed by a steady decline. In the data linkage study in Australia, the proportional increases of cancer risk after CT exposure were smaller based on the longer lag period compared with the 1-year lag period (5-year lag period: IRR=1.21, 95% CI=1.16–1.26; 10-year lag period: IRR=1.18, 95% CI=1.11–1.24). Shilnikova et al (2003) reported that the risk of leukaemia 3–5 years after radiation exposure was higher than that for later periods in an analysis of data among workers at the Mayak nuclear complex in Russia. For this issue, the BEIR VII committee supposed that the excess risk in the period 2–5 years after radiation exposure is similar to that observed 5 years later (National Research Council, 2006). So far, there is uncertainty in the magnitude of the risk during the follow-up period after radiation exposure. One possible explanation of the trend of decreased risk with longer follow-up is that the early increased risk led to a compensatory decrease in the following year. However, the true causes are unknown and could be a source of ongoing research.
The relatively large sample size, obtained from nation-wide population-based data sets, may increase the statistical power of our examinations of the associations between paediatric CT of the head and subsequent risk of malignancies and benign brain tumour. Widespread coverage of the nation-wide database allows the tracing of nearly all of the participants’ medical service-use histories. Furthermore, it is very uncommon for children to leave Taiwan for cancer care.
A limitation of this study was the difficulty in defining the actual radiation exposure from each CT scan because of variations in equipment types, shape and size of the head, the use of contrast, protocols of scanning, and purpose of examinations, which were not available in the NHRI data sets. Thus, we could not show dosimetry data and estimate dose–response relationships. We did not include radiation doses from other common medical exposures, such as plain radiographs, CT of the chest, and CT of the abdomen, in our analyses. Their exclusion is unlikely to have introduced major bias, because the radiation exposure to the head from such scans is typically considerably smaller than those for CT scans of the head. However, the effect of radiation on non-head cancer risk is underestimated in the present study. Thus, our result of no elevated risk of leukaemia and non-brain solid cancers should be interpreted with caution. It is only true after paediatric head CT scans. It remains unclear whether other types of medical radiation exposure increase the risk of leukaemia and non-brain solid cancers or not. Another limitation was that the exposure frequency might also have been underestimated because contracted Taiwan National Health Insurance (TNHI) practitioners collected the data from the head CT database, excluding non-TNHI data (including self-paying patients). In addition, data on head CT received before 1996 were unavailable in the NHRI data sets. This study also lacks information regarding the purpose of the CT scans. The NHIRD database does not provide this information, and we could not include the analysis to examine their association with subsequent cancer risk. Thus, it is possible to have screening effect and selection bias. To rule out those who underwent CT examination for precancerous conditions, we excluded children with disorders that might increase cancer risk and any cancer history. Furthermore, the lag period of 2 years might minimise the screening effect and the selection bias. Similarly, we could not provide more details regarding the histology subtypes of the malignant and benign brain tumours, although the patients who were included in the CICD were histologically or cytologically confirmed. We identified events using ICD-9 coding system in the CICD. For example, ICD-9-191 represented malignant neoplasm of the brain, but we could not further identify histology subtypes in this coding system.
In conclusion, the risk of developing benign brain tumours in Taiwan is 2.97 times higher among patients who underwent head CT scanning procedures during childhood than in those who did not receive CT scans during childhood. In particular, patients who underwent more frequent head CT scans are at a higher subsequent risk of overall cancer, leukaemia, and benign brain tumour. A large-scale study with longer follow-up is necessary to confirm this result.
References
Bednarz B, Athar B, Xu XG (2010) A comparative study on the risk of second primary cancers in out-of-field organs associated with radiotherapy of localized prostate carcinoma using Monte Carlo-based accelerator and patient models. Med Phys 37: 1987–1994.
Boice JD Jr, Blettner M, Kleinerman RA, Stovall M, Moloney WC, Engholm G, Austin DF, Bosch A, Cookfair DL, Krementz ET (1987) Radiation dose and leukemia risk in patients treated for cancer of the cervix. J Natl Cancer Inst 79: 1295–1311.
Brenner D, Elliston C, Hall E, Berdon W (2001) Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol 176: 289–296.
Brenner DJ, Hall EJ (2007) Computed tomography-an increasing source of radiation exposure. N Engl J Med 357: 2277–2284.
Chen YC, Wu JC, Chen TJ, Wetter T (2011) Reduced access to database. A publicly available database accelerates academic production. BMJ 342: d637.
Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML (2011) Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 20: 236–242.
Curtis RE, Boice JD Jr, Stovall M, Bernstein L, Greenberg RS, Flannery JT, Schwartz AG, Weyer P, Moloney WC, Hoover RN (1992) Risk of leukemia after chemotherapy and radiation treatment for breast cancer. N Engl J Med 326: 1745–1751.
Curtis RE, Boice JD Jr, Stovall M, Bernstein L, Holowaty E, Karjalainen S, Langmark F, Nasca PC, Schwartz AG, Schymura MJ, Storm HH, Toogood P, Weyer P, Moloney WC (1994) Relationship of leukemia risk to radiation dose following cancer of the uterine corpus. J Natl Cancer Inst 86: 1315–1324.
Fazel R, Krumholz HM, Wang Y, Ross JS, Chen J, Ting HH, Shah ND, Nasir K, Einstein AJ, Nallamothu BK (2009) Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med 361: 849–857.
Huang WY, Lin CC, Jen YM, Lin KT, Yang MH, Chen CM, Chang YN, Sung FC, Kao CH (2012) Association between adult otitis media and nasopharyngeal cancer: a nation-wide population-based cohort study. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology 104: 338–342.
Inskip PD, Kleinerman RA, Stovall M, Cookfair DL, Hadjimichael O, Moloney WC, Monson RR, Thompson WD, Wactawski-Wende J, Wagoner JK et al (1993) Leukemia, lymphoma, and multiple myeloma after pelvic radiotherapy for benign disease. Radiat Res 135: 108–124.
Ivanov VK, Kashcheev VV, Chekin SY, Maksioutov MA, Tumanov KA, Vlasov OK, Shchukina NV, Tsyb AF (2012) Radiation-epidemiological studies of thyroid cancer incidence in Russia after the Chernobyl accident (estimation of radiation risks, 1991-2008 follow-up period). Radiat Prot Dosimetry 151: 489–499.
Kao CH, Sun LM, Chen PC, Lin MC, Liang JA, Muo CH, Chang SN, Sung FC (2013) A population-based cohort study in Taiwan-use of insulin sensitizers can decrease cancer risk in diabetic patients? Ann Oncol 24: 523–530.
Liang JA, Sun LM, Lin MC, Chang SN, Sung FC, Muo CH, Kao CH (2012) A population-based nested case-control study in taiwan: use of 5alpha-reductase inhibitors did not decrease prostate cancer risk in patients with benign prostate hyperplasia. Oncologist 17: 986–991.
Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, Giles GG, Wallace AB, Anderson PR, Guiver TA, McGale P, Cain TM, Dowty JG, Bickerstaffe AC, Darby SC (2013) Cancer risk in 680 000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 346: f2360.
Mettler FA Jr, Bhargavan M, Faulkner K, Gilley DB, Gray JE, Ibbott GS, Lipoti JA, Mahesh M, McCrohan JL, Stabin MG, Thomadsen BR, Yoshizumi TT (2009) Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources-1950-2007. Radiology 253: 520–531.
National Council on Radiation Protection and Measurements, Scientific Commitee 6-2 on Radiation Exposure of the U.S. Population (2009) Ionizing radiation exposure of the population of the United States: recommendations of the National Council on Radiation Protection and Measurements. National Council on Radiation Protection and Measurements: Bethesda, MD, USA.
National Research Council (2006) Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII, Phase 2. National Academies Press: Washington, DC, USA.
Newhauser WD, Durante M (2011) Assessing the risk of second malignancies after modern radiotherapy. Nat Rev Cancer 11: 438–448.
Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, Howe NL, Ronckers CM, Rajaraman P, Sir Craft AW, Parker L, Berrington de Gonzalez A (2012) Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 380: 499–505.
Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K (2007) Solid cancer incidence in atomic bomb survivors: 1958-1998. Radiat Res 168: 1–64.
Shilnikova NS, Preston DL, Ron E, Gilbert ES, Vassilenko EK, Romanov SA, Kuznetsova IS, Sokolnikov ME, Okatenko PV, Kreslov VV, Koshurnikova NA (2003) Cancer mortality risk among workers at the Mayak nuclear complex. Radiat Res 159: 787–798.
Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, Berrington de Gonzalez A, Miglioretti DL (2009) Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 169: 2078–2086.
Swerdlow AJ, Douglas AJ, Hudson GV, Hudson BV, Bennett MH, MacLennan KA (1992) Risk of second primary cancers after Hodgkin's disease by type of treatment: analysis of 2846 patients in the British National Lymphoma Investigation. BMJ 304: 1137–1143.
United Nations Scientific Committee on the Effects of Atomic Radiation (2010) Sources and effects of ionizing radiation: United Nations Scientific Committee on the Effects of Atomic Radiation: UNSCEAR 2008 report to the General Assembly, with scientific annexes. United Nations: New York, NY, USA.
Weiss HA, Darby SC, Doll R (1994) Cancer mortality following X-ray treatment for ankylosing spondylitis. Int J Cancer 59: 327–338.
Weiss HA, Darby SC, Fearn T, Doll R (1995) Leukemia mortality after X-ray treatment for ankylosing spondylitis. Radiat Res 142: 1–11.
Acknowledgements
The study was supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center for Excellence (DOH102-TD-B-111-004), Taiwan Ministry of Health and Welfare Cancer Research Center for Excellence (MOHW103-TD-B-111-03), and International Research-Intensive Centers of Excellence in Taiwan (I-RiCE) (NSC101-2911-I-002-303).
Author Contributions
Study concept and design: W-Y Huang, C-H Kao; acquisition of data: C-Y Lin, Y-M Jen, M-H Yang, J-C Lin; analysis and interpretation of data: W-Y Huang, C-H Muo, C-H Kao; drafting of the manuscript: W-Y Huang, C-H Muo, C-H Kao; critical revision of the manuscript for important intellectual content: W-Y Huang, C-H Muo, C-H Kao; statistical analysis C-H Muo, F-C Sung; obtained funding: F-C Sung, C-H Kao; administrative, technical, or material support: F-C Sung; study supervision: C-H Kao.
Disclaimer
Taiwan Ministry of Health and Welfare had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. The interpretations and conclusions contained in this article do not represent those of the Bureau of National Health Insurance, Department of Health, or the NHRI. National Health Insurance Research Database, Taiwan. http://www.nhri.org.tw/nhird/en/index.html.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Huang, WY., Muo, CH., Lin, CY. et al. Paediatric head CT scan and subsequent risk of malignancy and benign brain tumour: a nation-wide population-based cohort study. Br J Cancer 110, 2354–2360 (2014). https://doi.org/10.1038/bjc.2014.103
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2014.103