Abstract
Background:
Tumour-associated stroma has a critical role in tumour proliferation. Our aim was to determine a specific protein expression profile of stromal angiogenic cytokines and matrix metalloproteinases (MMPs) to identify potential biomarkers or new therapy targets.
Methods:
Frozen tissue of primary colorectal cancer (n=25), liver (n=25) and lung metastases (n=23) was laser-microdissected to obtain tumour epithelial cells and adjacent tumour-associated stroma. Protein expression of nine angiogenic cytokines and eight MMPs was analysed using a multiplex-based protein assay.
Results:
We found a differential expression of several MMPs and angiogenic cytokines in tumour cells compared with adjacent tumour stroma. Cluster analysis displayed a tumour-site-dependent stromal expression of MMPs and angiogenic cytokines. Univariate analysis identified stromal MMP-2 and MMP-3 in primary colorectal cancer, stromal MMP-1, -2, -3 and Angiopoietin-2 in lung metastases and stromal MMP-12 and VEGF in liver metastases as prognostic markers (P>0.05, respectively). Furthermore, stroma-derived Angiopoietin-2 proved to be an independent prognostic marker in colorectal lung metastases.
Conclusion:
Expression of MMPs and angiogenic cytokines in tumour cells and adjacent tumour stroma is dependent on the tumour site. Stroma-derived MMPs and angiogenic cytokines may be useful prognostic biomarkers. These data can be helpful to identify new agents for a targeted therapy in patients with colorectal cancer.
Similar content being viewed by others
Main
Malignant tumours consist of a complex structure that is composed of malignant cancer cells and the surrounding tumour microenvironment. The tumour microenvironment contains a heterogenous group of stromal cells such as fibroblasts, endothelial cells, pericytes and inflammatory cells, which are embedded in the extracellular matrix (Sund and Kalluri, 2009). Via a continuous cross-talk between cancer cells and their cellular and extracellular microenvironment, tumour stroma exerts substantially influence on tumour progression and dissemination (Gout and Huot, 2008). However, the tumour microenvironment has a bimodal role in cancer: on one hand, it notably supports cancer cells in proliferation or forming new blood vessels for nutritive supply by releasing pro-tumorigenic cytokines and angiogenic factors (Kessenbrock et al, 2010; Sakurai and Kudo, 2011). On the other hand, tumour-associated stroma cells can release MMPs or tumour-inhibiting cytokines, which have rather a protective role against tumour growth and metastasis (Noel et al, 2012). Therefore, tumour stroma-derived angiogenic factors and MMPs might be promising targets for cancer therapy and could be utilised as a potential source for substantial biomarkers. Recent studies have reported that a stromal gene signature predicts clinical outcome in primary breast cancer and esophageal adenocarcinoma (Finak et al, 2008; Farmer et al, 2009; Saadi et al, 2010). Thus, stroma-derived expression signatures might complement clinical staging for risk stratification in patients with cancer, hence aiding to develop a ‘tailored’ therapy for an improved clinical outcome. Moreover, stroma-derived expression signatures may help to identify target genes, which would be suitable for new treatment approaches. Recent studies have solely focused on the expression profile on mRNA transcript level. The complex interactions between MMPs and angiogenic cytokines, however, are mainly modulated on the protein level (Gialeli et al, 2011). In this study, we performed a protein analysis using the new technology of a multiplex-based angiogenic cytokine and MMP assay. This assay allowed us to quantify the expression of nine angiogenic cytokines and eight MMPs in laser-microdissected tissue samples from colorectal cancer cells and adjacent tumour stroma. We thereby provided a detailed angiogenic protein profile for primary colorectal cancer, colorectal liver metastases and colorectal lung metastases.
Materials and methods
Patient characteristics and data collection
Tissue collection was approved by the Ethics Committee of the University of Heidelberg. A written informed consent for the tissue sampling was obtained preoperatively from all patients and the planned analyses regarding potential prognostic markers. For analysis, kryo-frozen tissue samples were retrieved from 25 primary colorectal adenocarcinomas, 25 colorectal liver metastases and 23 colorectal lung metastases. All samples were obtained from different individuals. Patients with primary colorectal cancer or colorectal metastases underwent tumour resection between 2004 and 2009 at the Department of General, Visceral, and Transplantation Surgery, University of Heidelberg. Patients with colorectal lung metastases were operated at the Department of Thoracic Surgery, University of Heidelberg between 2003 and 2008. Clinical information was obtained for all patients including variables such as age, gender, TNM classification, grading, tumour location (in case of primary colorectal cancer) and cancer-specific survival (time from diagnosis to cancer-related death or last follow-up).
Clinical specimens
Immediately after resection, samples were snap-frozen in liquid nitrogen and stored at −80 °C until further processing. A 10-μm reference section of each sample was cut and stained with hematoxylin and eosin by standard methods to evaluate the proportion of tumour tissue and adjacent tumour stroma. Samples with a tumour stroma proportion >30% were included in this study.
Microdissection
Tissue preparation and laser microdissection
Twenty-micrometre sections were cut from the frozen tissue using a cryostat (Leica, Wetzlar, Germany), mounted on Zeiss membrane slides (Carl Zeiss microimaging, Jena, Germany), stained with cresyl violet using an LCM Staining Kit (Ambion/Applied Biosystems, Darmstadt, Germany) and stored at −80 °C until further processing. Tumour (40 mm2) tissue and adjacent tumour stroma (40 mm2) were microdissected using a PALM Microbeam (Carl Zeiss microimaging). Microdissected tissue was transferred to an adhesive cap (Carl Zeiss) and stored immediately at −80 °C until further processing.
Protein lysates
Microdissected tissue samples were lysed in Bio-PlexLysis Buffer, and protein concentrations were evaluated as reported recently (Halama et al, 2011). Briefly, concentration of all lysates was determined using a BCA protein assay kit (Thermo Scientific, 58239 Schwerte, Germany). Subsequently, samples were adjusted to a total protein concentration of 150 μg ml−1 and quantified using the BioRadBio-Plex Human Angiogenesis Assay (Bio-Rad Laboratories, Inc., Hercules, CA 94547, USA) and the Millipore MILLIPLEX MAP Human MMP Panels 1 and 2 (Millipore, 290 Concord Road, Billerica, MA, USA) according to the manufacturer’s instructions. These panels included the following proteins: angiopoietin-2, follistatin, granulocyte colony-stimulating factor (G-CSF), hepatocyte growth factor (HGF), interleukin-8 (IL-8), leptin, platelet-derived growth factor beta (PDGF-BB), platelet endothelial cell adhesion molecule-1 (PECAM-1), vascular endothelial growth factor (VEGF), matrix metalloproteinase (MMP)-1, -2, -3, -7, -9, -10, -12 and -13. Standard curves and concentrations were calculated with Bio-Plex Manager 4.1.1 on the basis of the 5-parameter logistic plot regression formula. The detection sensitivity of all analysed samples ranged from 2 pg ml−1 to 30 ng ml−1.
Statistical analysis
The statistical analysis is based on the log-transformed values of MMPs and angiogenetic cytokines. Wilcoxon rank sum tests were used to test the pairwise associations of MMPs and angiogenetic cytokines. Pairwise signed-ranked Wilcoxon tests were implemented to analyse association between tumour and stromal tissue. P-values were adjusted for multiple testing using Hochberg’s method (Hochberg, 1988). A cluster analysis was used to identify patient groups with similar expression patterns. For cluster analysis, the distance between two clusters was measured by Ward’s method (Ward, 1963). Heatmaps including dendograms visualise the expression values and clustering of the patients. The influence of single MMPs and angiogenetic cytokines on cancer-specific survival was assessed by univariate analysis using the log-rank test. The Cox proportional hazards regression model was conducted on all covariates that showed a significant association with cancer-specific survival in the univariate analysis. P-values ⩽0.05 were considered to be significant. The statistical analysis was performed using R version 2.14.0 and 2.15.3 (http://www.r-project.org) and SPSS version 21.0 (IBM, New York, NY, USA).
Results
Patient characteristics
In our study, we have included 25 specimens of primary colorectal cancer, 25 specimens of colorectal liver metastases and 23 tissue samples from colorectal lung metastases (Supplementary Table 1). The median age of patients at the time of tumour resection was 69 years. Forty-nine patients were male and 24 were female. Eleven patients with primary colorectal cancer were diagnosed at the UICC stage II, 11 patients at the UICC stage III and three patients with the UICC stage IV. Eight out of 25 patients with colorectal liver metastases were suffering from synchronous disease and 17 specimens were retrieved from metachronous liver metastases. All specimens of colorectal lung metastases were obtained from metachronous secondary tumour sites. Five patients with rectal cancer were treated preoperatively by radiochemotherapy and 12 patients with primary colorectal cancer received postoperative adjuvant treatment. In 10 out of 25 patients with colorectal liver metastases, preoperative chemotherapy was applied within 3 months before liver surgery. All patients with lung metastases were chemotherapy-naive 3 months prior to surgical resection.
Stromal vs epithelial expression of MMPs and angiogenic cytokines
We started our approach by analysing the stromal vs epithelial expression of eight MMPs and nine angiogenic cytokines in primary colorectal cancer, and colorectal liver and lung metastases (Figure 1 and Table 1).
Twenty-five samples of primary colorectal cancer (CRC), 25 samples of colorectal liver metastases (liver) and 23 samples of colorectal lung metastases (lung) were subjected to laser microdissection to obtain separately tumour and stroma tissues. After lysis, protein expression was determined using the new technology of a multiplex-based angiogenic cytokine and MMP assay. Expression analysis included angiopoietin-2, follistatin, G-CSF, HGF, IL-8, leptin, PDGF-BB, PECAM-1, VEGF, MMP-1, -2, -3, -7, -9, -10, -12 and -13 in tumour epithelial cells (red) and tumour-associated stroma (green). Unit: pg ml−1. Each dot represents a single analysis of one tumour/stroma sample. P-values were adjusted for multiple testing using Hochberg’s method. *P<0.05.
Significantly higher expression between tumour and tumour stroma were found:
in all three tumour sites:
-
for tumour-derived VEGF
-
for stroma-derived angiopoietin-2, HGF and MMP-3.
in primary colorectal cancer and lung metastases:
-
for stroma-derived follistatin, G-CSF, PECAM-1, MMP-1 and -2
in lung and liver metastases:
-
for tumour-derived IL-8
only in primary colorectal cancer:
-
for tumour-derived PDGF
-
for stroma-derived leptin
only in colorectal lung metastases:
-
for stroma-derived MMP-10
only in colorectal liver metastases:
-
for tumour-derived leptin.
-
for stroma-derived MMP-9
MMP-12 and MMP-13 were not differentially expressed between tumour cells and the stromal compartment in all three tumour sites. In colorectal liver metastases, MMP-13 was below the detection threshold in the tumour compartment in all samples and in the stromal compartment in 23 out of 25 samples.
Tumour site-dependent expression of MMPs and angiogenic factors
In a second approach, we compared the expression profile of MMPs and angiogenic factors in relation to the tumour site. Interestingly, this analysis revealed a heterogenous expression signature of single MMPs and angiogenic cytokines between primary colorectal cancer, and colorectal liver and lung metastases (Figure 1). Significant differential expression (P<0.05) was found (Table 2):
-
in primary colorectal cancer vs lung metastases:
-
for two stroma-derived MMPs and two tumour-derived MMPs
-
in primary colorectal cancer vs liver metastases:
-
for 12 stroma-derived factors and for 10 tumour-derived factors
-
in liver vs lung metastases:
-
for 10 stroma-derived factors and for 10 tumour-derived factors.
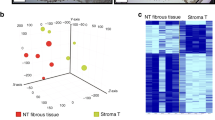
A cluster analysis of the expression of all tumoral and stromal MMPs and angiogenic cytokines was performed for all patients (Figure 2), revealing two cluster groups with a different expression profile. Cluster 1 included 21 out of 25 colorectal liver metastases and no sample from any other tumour site. Cluster 2 included all samples from primary colorectal cancer and lung metastasis as well as four liver metastasis. Cluster 1 was characterised by a higher expression of angiogenic cytokines and a lower expression of MMPs compared with cluster 2.
Tumour-site-dependent expression of MMPs and angiogenetic factors. Cluster analysis including all tumorous and stromal MMPs and angiogenic cytokines reveals a distinct expression pattern discriminating between primary colorectal cancer and lung metastases vs colorectal liver metastases. The expression pattern of colorectal liver metastases is characterised by a higher abundance of angiogenic cytokines and a lower expression of MMPs compared with primary colorectal cancer and lung metastases.
Expression of stromal MMPs and angiogenic cytokines in correlation with prognosis in patients with primary colorectal cancer, and liver and lung metastases
To evaluate an association between the stromal and tumour epithelial expression of angiogenetic factors/MMPs and clinical outcome, samples were dichotomised according to the median expression value of each single factor. Low expression was defined as protein levels lower or equal to the median value in contrast to a high expression where the protein level was higher than the median value. Univariate analysis by log-rank test revealed that low stromal expression of MMP-2 and MMP-3 was associated with a significantly shorter overall survival in primary colorectal cancer (Supplementary Table 1, Figure 3). For colorectal liver metastases, high expression of stroma-derived MMP-12 and stroma-derived VEGF correlated with a dismal prognosis (Supplementary Table 2, Figure 3). For colorectal lung metastases, high expression of stroma-derived MMP-1, MMP-2 and MMP-3 was a significant indicator for a more favourable clinical outcome, whereas high expression of stromal angiopoietin-2 was associated with a reduced cancer-specific survival (Supplementary Table 3, Figure 3). Finally, multivariate analysis by a Cox regression model for each tumour site proved angiopoietin-2 as an independent prognostic marker for cancer-specific survival in lung metastases (Table 3, Supplementary Tables 4 and 5) (hazard ratio stromal angiopoietin-2: 7.6; CI: 1.021–50.2 (P=0.048)).
Kaplan–Meier curves display cancer-specific survival in correlation with (A) stromal expression of MMP-2 in primary colorectal cancer, (B) stromal expression of MMP-3 in patients with primary colorectal cancer, (C) stromal expression of MMP-12 in patients with colorectal liver metastases, (D) stromal expression of VEGF in patients with colorectal liver metastases, (E) stromal expression of MMP-1 in patients with lung metastases, (F) stromal expression of MMP-2 in patients with lung metastases, (G) stromal expression of MMP-3 in patients with lung metastases, (H) stromal expression of angiopoietin-2 in patients with lung metastases.
Discussion
The continuous cross-talk between cancer cells and tumour microenvironment-associated cells has a significant role in tumour carcinogenesis and tumour progression. Apart from tumour cells, the tumour microenvironment is a crucial source of angiogenic cytokines, proteases and vascular stimulating factors, which are important to maintain the intercellular communication (Kalluri and Zeisberg, 2006; Orimo and Weinberg, 2006). This is the first study describing and quantifying a distinct protein expression profile of angiogenic cytokines and MMPs in tumour cells and surrounding tumour stroma of primary colorectal cancer as well as liver and lung metastases. Our results confirm the findings of several previous studies assessing the mRNA expression pattern of MMPs and angiogenic factors in colorectal cancer cells and adjacent stroma. For example, Poulsom et al (1992) and Chan et al (2001) described a strong stromal expression of MMP-2 in primary colorectal cancer. Likewise, RNA in situ hybridisation against MMP-9 revealed an abundant expression in stroma-associated macrophages in primary colorectal cancer but only a low expression in corresponding liver metastases (Illemann et al, 2006). In contrast, mRNA expression of VEGF is mainly confined to colorectal cancer cells in comparison to stromal cells (Wong et al, 1999). These studies are in good accordance with our protein expression results and show a good correlation between mRNA expression and protein expression.
As one of our main findings, we provide evidence that, besides tumour cells, the tumour-associated stroma is a relevant origin of angiogenic cytokines and MMPs in primary colorectal cancer and metastases. We show that MMP-3, Angiopoietin-2 and HGF are upregulated in adjacent tumour stroma compared with tumour cells in all tumour sites. Moreover, this is the first study revealing that the expression profiles of several angiogenic cytokines and MMPs are dependent on the tumour site. Intriguingly, there is a strong overlap of the expression signature of primary colorectal cancer and lung metastases, whereas colorectal liver metastases display a distinct expression pattern with >10 differentially regulated angiogenic cytokines/MMPs compared with the two other tumour sites. This finding may be of important relevance in the context of a potential targeted therapy against angiogenic cytokines or MMPs. In fact, anti-VEGF treatment by bevacizumab has an important role in first-line treatment of metastatic colorectal cancer (Macedo et al, 2012). Our data give evidence that VEGF is increased in tumour cells of colorectal liver metastases by an approximate two-fold change compared with lung metastases and primary colorectal cancer, respectively. Likewise, stromal angiopoietin-2, which is currently tested as a target for antitumour in clinical trials (Mita et al, 2010; Karlan et al, 2012), shows an approximately four-fold increase in colorectal liver metastases compared with primary colorectal cancer, and a two-fold upregulation in liver metastases vs lung metastases. In conclusion, the tumour-site expression analysis of potential therapeutical target cytokines might be useful to refine the individual treatment of patients with colorectal cancer in the context of a ‘tailored’ therapy.
Finally, we have evaluated the relation between stroma- and tumour-derived angiogenic cytokines/MMPs and prognosis. Although our patient cohort for each tumour site encompasses only a small number, we were able to observe a significant correlation between several MMPs/angiogenic cytokines and cancer-specific survival by univariate analysis. High expression levels of stromal MMP-2 and MMP-3 were indicators for an improved clinical outcome in patients with primary colorectal cancer and lung metastases. These data are in good accordance with two previous studies showing that low expression levels of MMP-2 and MMP-3 are adverse prognostic markers in primary colorectal cancer (Wong et al, 2011; Agesen et al, 2012). Moreover, our data show that upregulated stroma-derived MMP-1 is associated with a more favourable outcome in patients with lung metastases by univariate analysis. Initially, MMPs have been considered to elicit mainly pro-tumorigenic effects by degrading the extracellular matrix, hence facilitating tumour cell migration and invasion (Kessenbrock et al, 2010). However, more recent experimental evidence imply that some members of the MMP family may also exert tumour-suppressive functions (Decock et al, 2011; Noel et al, 2012). In conclusion, it is tempting to hypothesise that stromal overexpression of some members of the MMP family is part of the antitumour response and may contribute to a protective microenvironment of the host against cancer cells. This might also explain, why many clinical trials in the last decade have failed when broad-spectrum MMP inhibitors were applied to patients in an attempt to find an anticancer agent (Coussens et al, 2002). However, several tumour-recruited stroma cells also sustain tumour growth and promote tumour progression by tumour angiogenesis. This may explain our findings that overexpression of stromal VEGF in colorectal liver metastases relates to shortened clinical outcome. Moreover, we have identified high expression of stroma-derived angiopoietin-2 as an independent adverse prognostic marker in colorectal lung metastases by univariate and multivariate analyses. Angiopoietin-2 is expressed primarily by endothelial cells where it may increase tumour metastasis by promoting endothelial disruption, increasing tumour cell translocation and homing to target organs (Falcon et al, 2009; Holopainen et al, 2012). Our results complement data of a previous study, where serum angiopoietin-2 has been identified as a biomarker for reduced survival in patients with colorectal cancer (Goede et al, 2010) and was mainly expressed in the stromal compartment of colorectal cancer tissue (Goede et al, 2010). In summary, this is the first study using a comprehensive protein expression analysis to elucidate the expression signature of angiogenic cytokines and MMPs in tumour cells and tumour-associated stromal cells in primary colorectal cancer as well as colorectal liver and lung metastases. We showed a differential expression of several MMPs and angiogenic cytokines in tumour cells compared with tumour-associated stroma. Moreover, we provide evidence that the tumour site-related expression profile in colorectal liver metastases differs significantly from the expression profiles found in the primary colorectal cancer and colorectal lung metastases. Decreased expression of several stromal MMPs was associated with an inferior clinical outcome in primary colorectal cancer, lung metastases and liver metastases. Furthermore, we have identified stroma-derived angiopoietin-2 as an independent prognostic marker in colorectal lung metastases. However, further prospective studies including a larger size of patients are required to validate this hypothesis.
Change history
21 January 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Agesen TH, Sveen A, Merok MA, Lind GE, Nesbakken A, Skotheim RI, Lothe RA (2012) ColoGuideEx: a robust gene classifier specific for stage II colorectal cancer prognosis. Gut 61 (11): 1560–1567.
Chan CC, Menges M, Orzechowski HD, Orendain N, Pistorius G, Feifel G, Zeitz M, Stallmach A (2001) Increased matrix metalloproteinase 2 concentration and transcript expression in advanced colorectal carcinomas. Int J Colorectal Dis 16 (3): 133–140.
Coussens LM, Fingleton B, Matrisian LM (2002) Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295 (5564): 2387–2392.
Decock J, Thirkettle S, Wagstaff L, Edwards DR (2011) Matrix metalloproteinases: protective roles in cancer. J Cell Mol Med 15 (6): 1254–1265.
Falcon BL, Hashizume H, Koumoutsakos P, Chou J, Bready JV, Coxon A, Oliner JD, McDonald DM (2009) Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am J Pathol 175 (5): 2159–2170.
Farmer P, Bonnefoi H, Anderle P, Cameron D, Wirapati P, Becette V, Andre S, Piccart M, Campone M, Brain E, Macgrogan G, Petit T, Jassem J, Bibeau F, Blot E, Bogaerts J, Aguet M, Bergh J, Iggo R, Delorenzi M (2009) A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med 15 (1): 68–74.
Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, Hallett M, Park M (2008) Stromal gene expression predicts clinical outcome in breast cancer. Nat Med 14 (5): 518–527.
Gialeli C, Theocharis AD, Karamanos NK (2011) Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 278 (1): 16–27.
Goede V, Coutelle O, Neuneier J, Reinacher-Schick A, Schnell R, Koslowsky TC, Weihrauch MR, Cremer B, Kashkar H, Odenthal M, Augustin HG, Schmiegel W, Hallek M, Hacker UT (2010) Identification of serum angiopoietin-2 as a biomarker for clinical outcome of colorectal cancer patients treated with bevacizumab-containing therapy. Br J Cancer 103 (9): 1407–1414.
Gout S, Huot J (2008) Role of cancer microenvironment in metastasis: focus on colon cancer. Cancer Microenviron 1 (1): 69–83.
Halama N, Braun M, Kahlert C, Spille A, Quack C, Rahbari N, Koch M, Weitz J, Kloor M, Zoernig I, Schirmacher P, Brand K, Grabe N, Falk CS (2011) Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res 17 (4): 678–689.
Hochberg Y (1988) A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75 (4): 800–802.
Holopainen T, Saharinen P, D'Amico G, Lampinen A, Eklund L, Sormunen R, Anisimov A, Zarkada G, Lohela M, Helotera H, Tammela T, Benjamin LE, Yla-Herttuala S, Leow CC, Koh GY, Alitalo K (2012) Effects of angiopoietin-2-blocking antibody on endothelial cell-cell junctions and lung metastasis. J Natl Cancer Inst 104 (6): 461–475.
Illemann M, Bird N, Majeed A, Sehested M, Laerum OD, Lund LR, Dano K, Nielsen BS (2006) MMP-9 is differentially expressed in primary human colorectal adenocarcinomas and their metastases. Mol Cancer Res 4 (5): 293–302.
Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6 (5): 392–401.
Karlan BY, Oza AM, Richardson GE, Provencher DM, Hansen VL, Buck M, Chambers SK, Ghatage P, Pippitt CH Jr., Brown JV 3rd, Covens A, Nagarkar RV, Davy M, Leath CA 3rd, Nguyen H, Stepan DE, Weinreich DM, Tassoudji M, Sun YN, Vergote IB (2012) Randomized, double-blind, placebo-controlled phase II study of AMG 386 combined with weekly paclitaxel in patients with recurrent ovarian cancer. J Clin Oncol 30 (4): 362–371.
Kessenbrock K, Plaks V, Werb Z (2010) Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141 (1): 52–67.
Macedo LT, Lima AB, Sasse AD (2012) Addition of bevacizumab to first-line chemotherapy in advanced colorectal cancer: a systematic review and meta-analysis, with emphasis on chemotherapy subgroups. BMC Cancer 12 (1): 89.
Mita AC, Takimoto CH, Mita M, Tolcher A, Sankhala K, Sarantopoulos J, Valdivieso M, Wood L, Rasmussen E, Sun YN, Zhong ZD, Bass MB, Le N, LoRusso P (2010) Phase 1 study of AMG 386, a selective angiopoietin 1/2-neutralizing peptibody, in combination with chemotherapy in adults with advanced solid tumors. Clin Cancer Res 16 (11): 3044–3056.
Noel A, Gutierrez-Fernandez A, Sounni NE, Behrendt N, Maquoi E, Lund IK, Cal S, Hoyer-Hansen G, Lopez-Otin C (2012) New and paradoxical roles of matrix metalloproteinases in the tumor microenvironment. Front Pharmacol 3: 140.
Orimo A, Weinberg RA (2006) Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle 5 (15): 1597–1601.
Poulsom R, Pignatelli M, Stetler-Stevenson WG, Liotta LA, Wright PA, Jeffery RE, Longcroft JM, Rogers L, Stamp GW (1992) Stromal expression of 72 kda type IV collagenase (MMP-2) and TIMP-2 mRNAs in colorectal neoplasia. Am J Pathol 141 (2): 389–396.
Saadi A, Shannon NB, Lao-Sirieix P, O'Donovan M, Walker E, Clemons NJ, Hardwick JS, Zhang C, Das M, Save V, Novelli M, Balkwill F, Fitzgerald RC (2010) Stromal genes discriminate preinvasive from invasive disease, predict outcome, and highlight inflammatory pathways in digestive cancers. Proc Natl Acad Sci USA 107 (5): 2177–2182.
Sakurai T, Kudo M (2011) Signaling pathways governing tumor angiogenesis. Oncology 81 (Suppl 1): 24–29.
Sund M, Kalluri R (2009) Tumor stroma derived biomarkers in cancer. Cancer Metastasis Rev 28 (1-2): 177–183.
Ward JH (1963) Hierarchical grouping to optimize an objective function. J Am Stat Assoc 58 (301): 236–244.
Wong JC, Chan SK, Schaeffer DF, Sagaert X, Lim HJ, Kennecke H, Owen DA, Suh KW, Kim YB, Tai IT (2011) Absence of MMP2 expression correlates with poor clinical outcomes in rectal cancer, and is distinct from MMP1-related outcomes in colon cancer. Clin Cancer Res 17 (12): 4167–4176.
Wong MP, Cheung N, Yuen ST, Leung SY, Chung LP (1999) Vascular endothelial growth factor is up-regulated in the early pre-malignant stage of colorectal tumour progression. Int J Cancer 81 (6): 845–850.
Acknowledgements
We thank Ludmila Umansky and Tina Lerchl for their excellent technical assistance for BioPlex Assays. This work was conducted within the framework of the Clinical Research Unit (KFO 227) ‘Colorectal cancer: From primary tumor progression towards metastases’ funded by the German Research foundation (DFG); (Grant No. WE 3548/4-1).
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Kahlert, C., Pecqueux, M., Halama, N. et al. Tumour-site-dependent expression profile of angiogenic factors in tumour-associated stroma of primary colorectal cancer and metastases. Br J Cancer 110, 441–449 (2014). https://doi.org/10.1038/bjc.2013.745
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.745
Keywords
This article is cited by
-
Estimation of salivary matrix metalloproteinases- 12 (MMP- 12) levels among patients presenting with oral submucous fibrosis and oral squamous cell carcinoma
BMC Oral Health (2021)
-
Serum MMP7, MMP10 and MMP12 level as negative prognostic markers in colon cancer patients
BMC Cancer (2016)
-
Evaluation of Angiopoietin-2 as a biomarker in gastric cancer: results from the randomised phase III AVAGAST trial
British Journal of Cancer (2016)
-
Management of resectable colorectal lung metastases
Clinical & Experimental Metastasis (2016)
-
Immunoexpression of metalloproteinase 14 and tissue inhibitor of metalloproteinase 2 in colorectal carcinomas and lymph node metastases
Comparative Clinical Pathology (2015)