Abstract
Background:
Pazopanib achieved the end point of clinical activity in pretreated patients with urothelial cancer in a single-group, phase 2 trial. The objective was to identify biological predictors of clinical benefit to pazopanib in these patients.
Methods:
EDTA blood samples were collected at baseline (T0) and after 4 weeks (T1) of treatment, together with radiological imaging in all 41 patients to analyse plasma circulating angiogenic factor levels by multiplex ELISA plates. Changes from T0 to T1 in marker levels were matched with response with the covariance analysis. Univariable and multivariable analyses evaluated the association with overall survival (OS), adjusted for prespecified clinical variables. Net reclassification improvement (NRI) tested the performance of the recognised Cox model.
Results:
Increasing IL8T1 level associated with lower response probability at covariance analysis (P=0.010). Both IL8T0 (P=0.019) and IL8T1 (P=0.004) associated with OS and the prognostic model, including clinical variables and IL8T1 best-predicted OS after backward selection. The NRI for this model was 39%.
When analysed as a time-varying covariate, IL8T1 level<80 pg ml−1 portended significantly greater response (∼80%) and 6-month OS (∼60%) probability than level⩾80.
Conclusion:
IL8-level changes during pazopanib allowed for a prognostic improvement and were associated with response probability.
Similar content being viewed by others
Main
Despite a remarkable incidence rate (fourth most common malignancy in men in United States), the progress in the therapeutic paradigm of urothelial cancer (UC), particularly in advanced stages, was stagnant in the last few decades (Gallagher et al, 2008). A paradigm shift is needed to advance the field. For patients with advanced disease and who have failed chemotherapy regimens, a variety of single-agent or combination therapies have yielded modest response rates and poor survival estimates. Although vinflunine is approved by the European Medicines Agency for progressive disease after platinum-based therapy, the US Food and Drug Administration has no approved agents (Sonpavde et al, 2010). A plethora of unsuccessful phase 2 trials of targeted compounds, either alone or combined with chemotherapy, was tested in UC at different clinical stages. With regards to the antiangiogenic setting, a compelling preclinical rationale fostered clinical research in the field, and results were reported with the use of compounds targeting the vascular-endothelial growth factor (VEGF) receptor axis, including sorafenib, aflibercept, sunitinib, everolimus, and bevacizumab (Dreicer et al, 2009; Gallagher et al, 2010; Twardowski et al, 2010; Bellmunt et al, 2011; Hahn et al, 2011; Choueiri et al, 2012; Seront et al, 2012; Balar et al, 2013; Galsky et al, 2013; Milowsky et al, 2013). Despite the negative results achieved in a small study sponsored by the National Cancer Institute in United States (Pili et al, 2013), pazopanib was active in our single-group, phase 2 study, whereby an objective response rate of 17.1% was achieved in heavily pretreated patients (Necchi et al, 2012). Taking together the results of these trials, an invariably uniform scenario can be drawn consisting of a rather low response rate ranging from 5 to 15%, and a small impact on expected progression-free (PFS) and overall survival (OS). Despite this, a small cohort of extreme responders could be identified by obtaining an incredibly long-term clinical benefit from antiangiogenic compounds. Paradigmatic examples are those observed in the sunitinib trial (one partial response (PR) lasting 24 months; Gallagher et al, 2010) and in the everolimus trial (one response duration of 26 months; Iyer et al, 2012; Milowsky et al, 2013).
This is the reason why further investigations on targeted agents should aim at identifying this class of long-term survivors for whom an antiangiogenic approach might have sense. In the absence of available tissue- and blood-based predictors, we aimed at evaluating circulating angiogenic factors (CAFs) over time in our phase 2 trial of pazopanib. Yet, the role of interleukin-8 (IL8) was anticipated (Necchi et al, 2012), and herein we present the full results of circulating biomarker analyses, matched with contextual computed tomography (CT)/positron emission tomography (PET) results. Refining the prognostic ability of recognised clinical factors could facilitate the proper selection of patients for conducting confirmatory trials with pazopanib in this disease as well as the interpretation of retrospective data from phase 2 studies with similar compounds.
Patients and methods
Forty-one patients having failed at least one platinum-based chemotherapy regimen were enrolled in a single-group, phase 2 trial of Pazopanib 800 mg orally daily until disease progression/unacceptable toxicity. Ten millilitres of EDTA plasma samples were collected at baseline and every 4 weeks until drug discontinuation, together with CT and PET/CT restaging. Samples were centrifuged for 20 min at 2200 r.c.f./4 °C and immediately stored at ⩽−20 °C. The amount of cCAFs, such as VEGF, serum VEGF receptor (VEGFR)-1 and -2, stem-cell factor (cKIT), IL6, IL8, and IL12, hepatocyte growth factor, and transforming growth factor-β (TGFβ), was determined at baseline (T0) and after 4 weeks of treatment (T1) using commercially available ELISA kits (R&D Systems Inc., Minneapolis, MN, USA), according to manufacturer’s protocols. Samples and standards were added to the wells of a microtitre plate and the different CAFs were captured by the specific antibodies immobilised to the wells of the plate. Successively, a horseradish peroxidase-conjugated detection antibody was added to detect the bound protein. After incubation, the wells were washed and the antigen complex bound to the well was detected by addiction of tetramethylbenzidine substrate solution, and a blue colour developed in proportion to the amount of the biomarker present in the sample. Colour development was then stopped, turning the colour in the wells to yellow. The absorbance of the colour was measured at 450 nm, producing a signal that is proportional to the amount of the biomarker bound. Response Evaluation Criteria for Solid Tumors (RECIST) version 1.1 were used to evaluate objective response – primary end point of the trial (Eisenhauer et al, 2009). Patients were categorised as responders (complete response (CR), PR, including stable disease (SD)) or non-responders for study purposes. Metabolic responses were based on the European Organisation for Research and Treatment of Cancer criteria (Young et al, 1999). The PET CR was defined by the fluorodeoxyglucose (FDG) uptake disappearance in all lesions detected at baseline, whereas PR was defined as a decrease of standard uptake value (SUV)max⩾25%. Non-responders were considered as those patients with either a SUVmax decrease<25% or any increase of FDG uptake, and the appearance of new focal FDG uptake(s) with anatomical confirmation. The metabolic response evaluation was assessed per patient by a blinded, referral nuclear medicine physician as the sum of SUVmax of the target lesions. Clinical protocol and the informed consents relative to clinical and biological study participation were approved by the Institutional Review Board of the Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy. All patients provided written informed consent before study entry.
Statistical methods
Statistical analyses focused on the investigation of CAFs as possible biomarkers. The association structure between biomarkers was investigated by estimating a Pearson’s partial correlation matrix, whereby correlations between pairs of biomarkers were adjusted for their associations with others.
Changes in CAF levels between T0 (baseline) and T1 (after 4 weeks of treatment) were tested by means of paired Student’s t-tests. To investigate whether T1 concentrations were associated with tumour response (either RECIST or PET), covariance analyses were adopted, which are known as the most efficient approach for the analysis of pre–post designs.
The prognostic effect of singly taken CAFs on OS was investigated using Cox proportional hazard regression models, with and without adjustment for clinical variables. For the sake of parsimony, such an adjustment was obtained by using the score of a separate Cox model including the following covariates, chosen on the basis of prior knowledge and previously used (Necchi et al, 2012): Eastern Cooperative Oncology Group performance status (0 versus ⩾1), presence of liver metastases, site of tumour primary (bladder versus upper tract), haemoglobin level at baseline (<10 versus ⩾10 g dl−1), and number of disease sites (1–2 versus >2). As regards the biomarkers, T0 levels for all CAFs and T1 levels only for those factors that changed significantly from baseline were modelled in the above analyses. Furthermore, for investigating CAF joint prognostic effect, the factors that achieved a Wald’s test P-value⩽0.10 in either unadjusted or adjusted analyses were entered into a multivariable Cox model. A backward elimination procedure was then used to identify the strongest prognostic biomarkers. Net reclassification improvement (NRI) was calculated to measure the improvement in 6-month prediction yielded by selected CAFs when added to clinical variables in multivariable Cox models. This index is a novel measure of model predictive performance that has been specifically recommended for the assessment of biomarkers (Pencina et al, 2012; Rapsomaniki et al, 2012). The NRI is calculated distinctly for event and non-event patients, and the two estimates are then averaged. For completeness, we report all these figures, although the average is more meaningful for clinical interpretation: the closer to one, the better the prognostic improvement towards a reference model, which, in our case, relies on information provided by clinical variables only.
The analyses were carried out using the SAS (SAS Institute Inc., version 9.2) and R 2.15.2 software (http://www.r-project.org/, last access September 30th, 2013). Statistical significance was set at the conventional two-sided 5% level.
Results
Updated clinical outcomes
Forty-one patients with UC and treated with at least one dose of pazopanib were enrolled in the study between February 2010 and July 2011. The majority of patients (51%) entered beyond the second line, 17.1% had a PR, and 51.2% had a clinical benefit. Median PFS and OS were 2.6 (95% CI, 1.7–3.7) and 4.7 months (95% CI, 4.2–7.3 months), respectively (Necchi et al, 2012).
There were two very long-term responders. The first patient (ID 04) had a PR lasting 32 months and an OS of 37.9 months, in spite of having poor prognostic features, namely, an upper tract UC in origin, third-line treatment for progressive bulky retroperitoneal disease, and the presence of isolated liver metastasis. The second patient (ID 12) had a bladder primary and was treated in second-line setting for disseminated nodal disease progressing after four cycles of cisplatin and gemcitabine. He obtained a durable SD of 19 months and the OS was 35.9 months.
Circulating biomarker assessment
Although some significant results were achieved when investigating the association structure between CAFs (Supplementary Table 1), no strong correlations (as quantified by a coefficient ρ>0.80) were detected. In particular, the highest levels of correlation were observed between VEGF and VEGFR2 (ρ=−0.48), VEGFR1 and IL8 (ρ=0.45), and VEGFR2 and TGFβ (ρ=0.48).
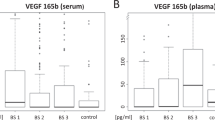
Table 1 and box plots of Figure 1 provide a descriptive analysis of pre–post pazopanib treatment biomarker levels. Significant T1–T0 modulation was observed for VEGF (P<0.001), VEGFR2 (P<0.001), cKIT (P<0.001), and IL8 (P=0.008). A 4-week increase was reported for VEGF and IL8 concentrations, whereas VEGFR2 and cKIT levels decreased. A separate description of T0–T1 CAFs changes in the two long-term responders is provided in Supplementary Table 2.
A significant association was detected between tumour RECIST response and IL8T1 levels (P=0.010 at covariance analysis). In detail, median IL8T0 levels were comparable between responders (67 pg ml−1) and non-responders (67.5 pg ml−1), whereas IL8T1 levels differed significantly between the two groups, being 69.8 pg ml−1 in responders (Figure 2A) and 97.6 pg ml−1 in non-responders (Figure 2B). As regards associations with PET response compared with baseline, significant results were achieved for IL12T0 levels (P=0.039 from covariance analysis) in contrast to IL8T0 and IL8T1 levels (P=0.111 each).
Table 2 shows the results of Cox model analyses focusing on the prognostic effects of singly taken CAFs on OS. Significant results were obtained for IL8T0 (P=0.015), IL8T1 (P=0.012), VEGFT1 (P=0.007), and TGFβT0 (P=0.038). After adjustment for the clinical variables, only IL8T0 (P=0.019) and IL8T1 (P=0.004) remained significant.
By jointly modelling IL8T1, IL8T0, VEGFT1, cKITT1, and TGFβT0, which are the biomarkers selected for the multivariable analysis, the average prognostic improvement over clinical variables (as quantified by NRI) was 60% (Table 3, model 1). Nevertheless, by applying a backward variable selection procedure, only IL8T1 retained its statistical significance. In this case, the average improvement in prediction was 39%.
By plotting the response probability and 6-month survival (Figure 3A and B) according to IL8T1 (and by adjusting for the fixed – median – value of IL8T0 at 67 pg ml−1), it turned out that a threshold of 80 pg ml−1 might be a reasonable cut-off value for prognostic discrimination; in particular, patients below the threshold show a relatively favourable prognosis for both outcomes.
Results by analysing IL8T1 level. Results by analysing IL8T1 level as a continuous time-varying covariate in association with response probability by the logistic regression model (A) and with 6-month survival probability by the Cox model (B). The model was adjusted for a fixed IL8T0 value of 67 pg ml−1 (median value).
Discussion
Our study reports a prognostic improvement by adding a biological variable to clinical parameters in the context of an antiangiogenic treatment.
There are a number of preclinical evidences supporting a role for angiogenesis in UC. Angiogenesis and VEGF possess key roles in UC initiation, progression, and invasion. Moreover, investigators have demonstrated an association between VEGF expression and prognosis of UC, as well as an improved tumour control with platinum-based chemotherapy plus antiangiogenic therapy in preclinical models (Dickinson et al, 1994; Jaeger et al, 1995; Wu et al, 2003). The present proof-of-principle trial showed a consistent pattern of cytokine reaction in UC patients treated with pazopanib and provided circumstantial evidences for the role of microenvironment as a framework of druggable targets in this disease.
To the best of our knowledge, this is the first time that the role of IL8 evaluated as dynamically in relation to response and outcome was obtained in the clinic. The most clinically sound observation was that patients with high baseline and 4-week levels of IL8, and, most importantly, those with rising serum levels of IL8 during pazopanib, particularly those with levels encompassing 80 pg ml−1 at 4 weeks of treatment, had a significantly lower chance of responding and 6-month survival probability. Interleukin-8 level at 4 weeks were then an independent prognostic factor for survival, together with recognised clinical variables.
Results should be taken with caution based on the limitations of the small sample size and the absence of a control arm. Moreover, when looking at the individual patient levels, it turned out that one of the two long-term survivors who achieved a prolonged SD with pazopanib had T1 levels rising to 100.0 pg ml−1 (Supplementary Table 2).
Rising levels of IL8 have been already associated with the development of resistance to antiangiogenic agent sunitinib in preclinical models of renal cell carcinoma, but this mechanistic association is hard to be unravelled based on present results (Huang et al, 2010). In fact, IL8 is produced by tumour cells of different histologies and raising serum concentrations of this chemokine were associated with tumour burden and increasing stage in a variety of solid neoplasms (Mian et al, 2003; Waugh and Wilson 2008; Britschgi et al, 2012; Lippitz, 2013). Hence, the question whether IL8 levels and their change over time might be solely a drug and tumour-induced epiphenomenon or rather a signal to allow selecting patients who are most likely to respond/survive remains unanswered. On the other hand, IL8 is a recognised mediator of tumour growth and metastatisation potential, and its role as a predictor of clinical benefit has been already reported in bladder cancer and renal cell carcinoma patients receiving sunitinib and pazopanib, respectively (Bellmunt et al, 2011; Tran et al, 2012). Furthermore, interleukin-8, as well as IL6, represents an activation of the immunostimulatory system and has been associated with a worse prognosis in cancer, independent of the tumour heterogeneity (Lippitz, 2013). Taken together, present observations and available knowledge might provide a rationale for the therapeutic role of agents targeting IL8 in UC, in combination or a sequence with a TKI. Among the available drugs, although the activity of the fully human anti-IL8 antibody ABX-IL8 was provided only in preclinical models (Mian et al, 2003), another anti-IL8 compound, reparixin (Dompè s.p.a.), is currently in phase 2 development in early breast cancer (ClinicalTrials.gov number NCT01861054).
Investigation on the prognostic contribution of IL8 should be pursued further in this disease, particularly in trials with antiangiogenic TKIs, to validate a potential tool for a patient-enrichment design. This could apply to two ongoing phase 2 trials of pazopanib combined with paclitaxel and gemcitabine, respectively, in UC (registered with ClinicalTrials.gov, number NCT01108055 and NCT01622660, respectively).
Although aberrations resulting in sensitivity to VEGFR-directed TKI might exist in the microenvironment rather than the tumour itself, this theory has yet to be proven. Yet, another signal corroborating the role of microenvironment in this setting was relative to the association of baseline levels of IL12 and metabolic response at 4 weeks (lower levels associated with PET response). Again, this observation should be cautiously unravelled, but a possible explanation may be that IL12 is an essential pro-inflammatory cytokine that is decreased in several cancer types, particularly in later stages; hence, it might be in relation to inflammatory and FDG-avid peri-tumoural tissue, the first to be dampened by an active targeted compound (Del Vecchio et al, 2007). Baseline TGFβ provided signals of prognostic effect. TGFβ is one of the principal immune-suppressive factors secreted by tumour cells and it possesses a huge spectrum of activity depending on the type of activated receptor (Bierie and Moses, 2006). A phase 2 trial is ongoing at our centre with the fully human monoclonal antibody directed against TGFβ receptor ALK1, PF03446962 (Pfizer Inc, La Jolla, CA, USA), a compound endowed with distinct antivascular activity, as second-line therapy in UC (ClinicalTrials.gov, number NCT01620970). Combined results from our group and from other clinical trials worldwide underscored the clinical meaning of targeting angiogenesis thus far, but an improvement in trial design based on patient selection/enrichment is desperately needed. Thus far, the sobering realisation of clinical trials with this class of agents was that of a small activity followed by resistance developing in a few months. Observations are hampered by the class activity of these drugs, not corresponding to tumour shrinkage for the majority of cases. A discrepancy was usually observed between an overall modest survival improvement and the existence of small subset of patients achieving an incredibly long-term response-stabilisation or even CR, far beyond what could be reasonably expected a priori. Going forward, an international cooperation to validate the present findings is required. The design of a multicentre data set, including CAFs from multiple cohorts of patients receiving anti-VEGF(R) compounds in phase 2 trials has the potential to render these results broadly applicable to antiangiogenic drugs in future clinical trials.
In conclusion, a caveat of present series is that tumour biology is suboptimally captured by clinical and laboratory features, such as those evaluated and the discovery of molecular predictors linked to an aggressive phenotype, and treatment resistance still needs a paradigm change. This is the reason why we are now moving towards a genomic profiling that yields a number of theoretical advantages over the former approach to guide informed clinical trials (Iyer et al, 2012). Extensive genomic profiling of tumour samples, particularly of defined subsets of patients who achieve extreme responses to antiangiogenic drugs, such as pazopanib, may allow for the identification of a landscape of novel druggable biomarkers.
Change history
07 January 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Balar AV, Apolo AB, Ostrovnaya I, Mironov S, Iasonos A, Trout A, Regazzi AM, Garcia-Grossman IR, Gallagher DJ, Milowsky MI, Bajorin DF (2013) Phase II study of gemcitabine, carboplatin, and bevacizumab in patients with advanced unresectable or metastatic urothelial cancer. J Clin Oncol 31: 724–730.
Bellmunt J, González-Larriba JL, Prior C, Maroto P, Carles J, Castellano D, Mellado B, Gallardo E, Perez-Gracia JL, Aguilar G, Villanueva X, Albanell J, Calvo A (2011) Phase II study of sunitinib as first-line treatment of urothelial cancer patients ineligible to receive cisplatin-based chemotherapy: baseline interleukin-8 and tumor contrast-enhancement as potential predictive factors of activity. Ann Oncol 22: 2646–2653.
Bierie B, Moses HL (2006) TGFβ: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer 6: 506–520.
Britschgi A, Andraos R, Brinkhaus H, Klebba I, Romanet V, Müller U, Murakami M, Radimerski T, Bentires-Alj M (2012) JAK2/STAT5 inhibition circumvents resistance to PI3K/mTOR blockade: a rationale for cotargeting these pathways in metastatic breast cancer. Cancer Cell 22: 796–811.
Choueiri TK, Ross RW, Jacobus S, Vaishampayan U, Yu EY, Quinn DI, Hahn NM, Hutson TE, Sonpavde G, Morrissey SC, Buckle GC, Kim WY, Petrylak DP, Ryan CW, Eisenberger MA, Mortazavi A, Bubley GJ, Taplin ME, Rosenberg JE, Kantoff PW (2012) Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. J Clin Oncol 30: 507–512.
Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, Anichini A (2007) Interleukin-12: biological properties and clinical application. Clin Cancer Res 13: 4677–4685.
Dickinson AJ, Fox SB, Persad RA, Hollyer J, Sibley GN, Harris AL (1994) Quantification of angiogenesis as an independent predictor of prognosis in invasive bladder carcinomas. Br J Urol 74: 762–766.
Dreicer R, Li H, Stein M, DiPaola R, Eleff M, Roth BJ, Wilding G (2009) Phase 2 trial of sorafenib in patients with advanced urothelial cancer: a trial of the Eastern Cooperative Oncology Group. Cancer 115: 4090–4095.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45: 228–247.
Gallagher DJ, Milowsky MI, Bajorin DF (2008) Advanced bladder cancer: status of first-line chemotherapy and the search for active agents in the second-line setting. Cancer 113: 1284–1293.
Gallagher DJ, Milowsky MI, Gerst SR, Ishill N, Riches J, Regazzi A, Boyle MG, Trout A, Flaherty AM, Bajorin DF (2010) Phase II study of sunitinib in patients with metastatic urothelial cancer. J Clin Oncol 28: 1373–1379.
Galsky MD, Hahn NM, Powles T, Hellerstedt BA, Lerner SP, Gardner TA, Yu M, O'Rourke M, Vogelzang NJ, Kocs D, McKenney SA, Melnyk AM Jr, Hutson TE, Rauch M, Wang Y, Asmar L, Sonpavde G (2013) Gemcitabine, cisplatin, and sunitinib for metastatic urothelial carcinoma and as preoperative therapy for muscle-invasive bladder cancer. Clin Genitourin Cancer 11: 175–181.
Hahn NM, Stadler WM, Zon RT, Waterhouse D, Picus J, Nattam S, Johnson CS, Perkins SM, Waddell MJ, Sweeney CJ Hoosier Oncology Group (2011) Phase II trial of cisplatin, gemcitabine, and bevacizumab as first-line therapy for metastatic urothelial carcinoma: Hoosier Oncology Group GU 04-75. J Clin Oncol 29: 1525–1530.
Huang D, Ding Y, Zhou M, Rini BI, Petillo D, Qian CN, Kahnoski R, Futreal PA, Furge KA, Teh BT (2010) Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res 70: 1063–1071.
Iyer G, Hanrahan AJ, Milowsky MI, Al-Ahmadie H, Scott SN, Janakiraman M, Pirun M, Sander C, Socci ND, Ostrovnaya I, Viale A, Heguy A, Peng L, Chan TA, Bochner B, Bajorin DF, Berger MF, Taylor BS, Solit DB (2012) Genome sequencing identifies a basis for everolimus sensitivity. Science 338: 221.
Jaeger TM, Weidner N, Chew K, Moore DH, Kerschmann RL, Waldman FM, Carroll PR (1995) Tumor angiogenesis correlates with lymph node metastases in invasive bladder cancer. J Urol 154: 69–71.
Lippitz BE (2013) Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol 14: 218–228.
Mian BM, Dinney CP, Bermejo CE, Sweeney P, Tellez C, Yang XD, Gudas JM, McConkey DJ, Bar-Eli M (2003) Fully human anti-interleukin 8 antibody inhibits tumor growth in orthotopic bladder cancer xenografts via down-regulation of matrix metalloproteases and nuclear factor-kB. Clin Cancer Res 9: 3167–3175.
Milowsky MI, Iyer G, Regazzi AM, Al-Ahmadie H, Gerst SR, Ostrovnaya I, Gellert LL, Kaplan R, Garcia-Grossman IR, Pendse D, Balar AV, Flaherty AM, Trout A, Solit DB, Bajorin DF (2013) Phase II study of everolimus in metastatic urothelial cancer. BJU Int 112: 462–470.
Necchi A, Mariani L, Zaffaroni N, Schwartz LH, Giannatempo P, Crippa F, Morosi C, Lanocita R, Sava T, Ortega C, Messina C, Sacco C, Pennati M, Daidone MG, Nicolai N, De Braud F, Gianni AM, Salvioni R (2012) Pazopanib in advanced and platinum-resistant urothelial cancer: an open-label, single group, phase 2 trial. Lancet Oncol 13: 810–816.
Pencina MJ, D’Agostino RB, Demler OV (2012) Novel metrics for evaluating improvement in discrimination: net reclassification and integrated discrimination improvement for normal variables and nested models. Stat Med 31: 101–113.
Pili R, Qin R, Flynn PJ, Picus J, Millward M, Ho WM, Pitot HC, Tan W, Miles KM, Erlichman C, Vaishampayan U (2013) A phase II safety and efficacy study of the vascular endothelial growth factor receptor tyrosine kinase inhibitor pazopanib in patients with metastatic urothelial cancer. Clin Genitourin Cancer pii (13): S1558–7673.
Rapsomaniki E, White IR, Wood AM, Thompson SG (2012) A framework for quantifying net benefis of alternative prognostic models. Stat Med 31: 114–130.
Seront E, Rottey S, Sautois B, Kerger J, D'Hondt LA, Verschaeve V, Canon JL, Dopchie C, Vandenbulcke JM, Whenham N, Goeminne JC, Clausse M, Verhoeven D, Glorieux P, Branders S, Dupont P, Schoonjans J, Feron O, Machiels JP (2012) Phase II study of everolimus in patients with locally advanced or metastatic transitional cell carcinoma of the urothelial tract: clinical activity, molecular response, and biomarkers. Ann Oncol 23: 2663–2670.
Sonpavde G, Sternberg CN, Rosenberg JE, Hahn NM, Galsky MD, Vogelzang NJ (2010) Second-line systemic therapy and emerging drugs for metastatic transitional-cell carcinoma of the urothelium. Lancet Oncol 11: 861–870.
Tran HT, Liu Y, Zurita AJ, Lin Y, Baker-Neblett KL, Martin AM, Figlin RA, Hutson TE, Sternberg CN, Amado RG, Pandite LN, Heymach JV (2012) Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol 13: 827–837.
Twardowski P, Stadler WM, Frankel P, Lara PN, Ruel C, Chatta G, Heath E, Quinn DI, Gandara DR (2010) Phase II study of aflibercept (VEGF-Trap) in patients with recurrent or metastatic urthelial cancer, a California Cancer Consortium trial. Urology 76: 923–926.
Waugh DJJ, Wilson C (2008) The interleukin-8 pathway in cancer. Clin Cancer Res 14: 6735–6741.
Wu W, Shu X, Hovsepyan H, Mosteller RD, Broek D (2003) VEGF receptor expression and signaling in human bladder tumors. Oncogene 22: 3361–3370.
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, Pruim J, Price P (1999) Measurement of clinical and subclinical tumour response using (18F)-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for the Research and Treatment of Cancer (EORTC) PET study group. Eur J Cancer 35: 1773–1782.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Presented in part at the 2012 Markers in Cancer Meeting, the 2012 ASCO Annual Meeting and the 2012 AACR Annual Meeting, 31 March–4 April 2012, Chicago, IL, USA.
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Necchi, A., Pennati, M., Zaffaroni, N. et al. Analysis of plasma cytokines and angiogenic factors in patients with pretreated urothelial cancer receiving Pazopanib: the role of circulating interleukin-8 to enhance the prognostic accuracy. Br J Cancer 110, 26–33 (2014). https://doi.org/10.1038/bjc.2013.719
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.719
Keywords
This article is cited by
-
[18F]Fluciclatide PET as a biomarker of response to combination therapy of pazopanib and paclitaxel in platinum-resistant/refractory ovarian cancer
European Journal of Nuclear Medicine and Molecular Imaging (2020)
-
Evaluation of efficacy and safety markers in a phase II study of metastatic colorectal cancer treated with aflibercept in the first-line setting
British Journal of Cancer (2015)