Abstract
Background:
Gamma-glutamyltransferase (GGT) – a membrane-bound enzyme crucially involved in the cell’s detoxification pathway and apoptotic balance – is involved in tumour development, progression and chemotherapy resistance. Elevated GGT serum levels are associated with increased cancer risk in women and worse prognosis in gynaecologic cancers. The present study investigated the prognostic role of GGT in ovarian cancer patients.
Methods:
In this multicenter study, pre-therapeutic GGT levels were ascertained in 634 consecutive patients with epithelial ovarian cancer (EOC, n=567) and borderline tumour of the ovary (BTO, n=67). Gamma-glutamyltransferase serum levels were associated with clinicopathological parameters and uni- and multivariate survival analyses were performed. Immunohistochemistry of GGT was performed in ovarian cancer tissue and correlated with GGT serum levels.
Results:
Pre-therapeutic GGT serum levels were higher in patients with EOC (28.56 (38.24) U l−1) than in patients with BTO (20.01 (12.78) U l−1, P=0.01). High GGT serum levels were associated with advanced FIGO stage (P<0.001) and with worse overall survival in univariate (P<0.001) and multivariable analysis (P=0.02, HR 1.2 (1.1–1.5)). We further investigated the association between systemic GGT serum levels and local GGT expression in EOC tumour tissue and observed an association between these two parameters (P=0.03).
Conclusion:
High pre-therapeutic GGT serum levels are associated with advanced tumour stage and serve as an independent prognostic marker for worse overall survival in patients with EOC. Gamma-glutamyltransferase expression in ovarian cancer tissue is reflected in GGT serum levels.
Similar content being viewed by others
Main
Gamma-glutamyltransferase (GGT) is a membrane-bound enzyme that has a crucial role in glutathione (GSH) metabolism. Glutathione metabolism is important for protecting cells against oxidants, which are produced during normal metabolism (Whitfield, 2001). Therefore, pathologic states of oxidative stress, such as carcinogenesis, lead to increased GSH and GGT levels (Whitfield, 2001). Glutathione is a marker for apoptotic balance, as it is crucial in the removal and detoxification of carcinogens, and alterations of this pathway can have a profound effect on cell survival (Hanigan, 1995; Dominici et al, 1999; Hanigan et al, 1999; Whitfield, 2001).
Gamma-glutamyltransferase expression is elevated in a variety of tumours (Hanigan, 1995; Hanigan et al, 1999; Franzini et al, 2006). Furthermore, GSH and GGT have been repeatedly described to have a role in tumour progression, invasion, and anticancer-drug resistance (Hanigan, 1995; Dominici et al, 1999; Hanigan et al, 1999; Franzini et al, 2006; Pompella et al, 2006; Corti et al, 2010). In a mouse model, GGT-positive tumours grew faster and were more likely to be resistant to platinum-containing chemotherapy subsequently leading to a worse prognosis (Hanigan et al, 1999). Recently, large epidemiologic studies revealed that plasma GGT was associated with an increased risk of developing cancer (Strasak et al, 2008a, 2008b; Fentiman and Allen, 2010; Van Hemelrijck et al, 2011). With respect to gynaecologic malignancies, increased GGT serum levels were associated with an increased risk for cervical cancer in a prospective epidemiological cohort study comprising 79 279 women (Strasak et al, 2008a). Moreover, preoperatively increased GGT serum levels were associated with advanced tumour stage in cervical cancer and with worse prognosis in endometrial cancer (Polterauer et al, 2011; Seebacher et al, 2012).
The present multicenter study investigated the association of preoperative GGT serum levels and survival in epithelial ovarian cancer (EOC) patients. To better characterise the role of GGT in ovarian cancer we also investigated GGT serum levels of borderline tumours of the ovary and the association between GGT serum levels and GGT expression in ovarian cancer tissue.
Materials and methods
Patients
We included 634 consecutive patients with EOC (n=567) and borderline tumour of the ovary (BTO) (n=67) treated between 1999 and 2008 (Department of General Gynecology and Gynecologic Oncology, Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria, n=314 and Department of Obstetrics and Gynecology, Innsbruck Medical University, Tirol, Austria, n=320). Clinical data were extracted from the respective Gynecologic Oncology Registries. Institutional review board approval was obtained prior to the study (IRB approval numbers: 266/2010 (Ethics Committee Medical University of Vienna) and UN4144 (Ethics Committee Medical University of Innsbruck)).
Prior to therapy, physical examination and blood tests were performed by a consultant in internal medicine, and the results were documented. Patients who presented with pre-existing co-morbidities, known to be related with elevation of GGT (i.e. hepato-biliary tract-, pancreatic- and heart disease or alcohol abuse) were not included in the study.
Clinical management
Patients were treated according to the guidelines at the respective institution with upfront surgery and adjuvant platinum-based chemotherapy. Surgery was performed according to FIGO (International Federation of Gynecologists and Obstetricians) guidelines (Benedet et al, 2000). All patients with tumour stages FIGO Ic to IV and all patients with clear cell carcinoma received a platinum-based chemotherapy.
Owing to very advanced disease, 32 (5.64%) patients received upfront diagnostic laparoscopy followed by three cycles of neoadjuvant platinum-based chemotherapy, debulking surgery as described above and three cycles of platinum-based chemotherapy.
Post-therapeutically, all patients were followed up using pelvic examination and serum tumour marker evaluation four times a year for years 1–3, twice a year for year 4–6 and once a year for years 7–10. Abdominal ultrasound examination and computer tomography (CT) thorax/abdomen were performed once a year for up to 10 years or when clinically indicated.
GGT measurement
As part of clinical routine, blood samples for the evaluation of serum GGT levels were obtained by peripheral venous puncture 24–48 h prior to therapy. Gamma-glutamyltransferase was routinely determined as a part of the preoperative work-up to rule out liver damage before treatment start. Gamma-glutamyltransferase concentrations were analysed with an enzyme kinetic assay (Modular Hitachi 747 and Hitachi 917, Roche Diagnostics, Vienna, Austria), as described previously (Kazemi-Shirazi et al, 2007).
GGT immunohistochemistry
Slides were cut and stored at room temperature prior to staining. Slides were deparaffinized, rehydrated and quenched for endogenous peroxidase. The retrieval was performed by microwaving the slides in EDTA (1 mM, pH 8.0). Immunostaining was performed using the UltraVision detection system according to the manufacturer’s protocol (Thermo Fisher Scientific, Hudson, NH, USA). Slides were incubated with primary polyclonal rabbit antibody against GGT7 (1 : 50; code HPA013204, Sigma-Aldrich, St Louis, MO, USA) at 4 °C overnight, followed by incubation with Primary Antibody Enhancer (Primary Antibody Enhancer, TL-015-PB, Thermo Fisher Scientific) and horseradish peroxidase (HRP) Polymer (HRP Polymer, TL-015-PH, Thermo Fisher Scientific). Slides were stained with diamino-benzidine (DAB) (DAB Chromogen 1 : 50 in DAB Substrate Buffer, K0673, Dako, Glostrup, Denmark) and counterstained with hematoxylin. Kidney tissue sections were used as a positive control, and rabbit immunoglobulin as a negative control. Gamma-glutamyltransferase expression levels were determined using a scoring system based on the intensity of staining (0–3) compared to the negative control (0). Samples were examined by three independent observers, including a gynaecological pathologist, whereby rescoring was conducted in samples with inconsistent scoring, leading to the following GGT expression groups: negative (0); weak (1); moderate (2) and strong (3) staining. For statistical analysis, classification into GGT-high and GGT-low was performed according to strong vs negative to moderate GGT expression levels, respectively.
Statistical analysis
Values are given as mean (standard deviation (s.d.)) when normally distributed or as median (interquartile range (IQR)) at presence of skewed distribution. Student’s t-test or Kruskal–Wallis tests were used to compare pre-therapeutic GGT serum levels and clinicopathological parameters depending on the distribution of GGT serum levels.
Survival probabilities were calculated by the product limit method of Kaplan and Meier. The results were analysed for the endpoint of overall survival. Survival times of patients still alive were censored with the last follow-up date. A multivariate Cox regression model for overall survival was performed, comprising FIGO stage (IV vs III vs II vs I), histological grade (G3 vs G2 vs G1), residual tumour (yes vs no), patient’s age at diagnosis (metric parameter) and GGT risk groups (D vs C vs B vs A). Patients were assigned to the previously described GGT cancer risk groups (8, 11, 12) as follows: GGT <17.99 U l−1: group A (normal low), 18.00–35.99 U l−1: group B (normal high), 36.00–71.99 U l−1: group C (elevated), and >72.00 U l−1: group D (highly elevated). Results of the multivariate survival analyses are provided as P-value (hazard ratio (HR) and 95% confidence interval (95% CI)). In an additional analysis, we evaluated the adjusted association of continuous GGT values with survival, using the technique of multivariable fractional polynomials to detect a potentially non-linear relationship (Royston and Altman, 1994). Furthermore, the discriminative ability of the multivariable model was assessed using a concordance index, which was based on leave-one-out cross-validated prognostic indices (Uno et al, 2011).
The association between the categorical variables, GGT tissue expression levels and GGT serum risk groups were calculated using Pearson χ2 test.
P-values of <0.05 were considered statistically significant. Statistical software SPSS 18.0 for Mac (SPSS 18.0, SPSS Inc, Chicago, IL, USA) and SAS and SPlus (Version 2000 Professional, Redmond, WA, USA) were used for statistical analysis.
Results
Demographic and clinical characteristics of the study cohort are shown in Table 1. All parameters are provided for patients with EOC only. Characteristics of patients with BTO are not provided. Patients with EOC have significantly higher pre-therapeutic GGT serum levels (28.56 (38.24) U l−1) compared to patients with BTO (20.01 (12.78) U l−1, P=0.01). Elevated pre-therapeutic GGT serum levels are associated with advanced tumour stage, but not with residual tumour, histological grade and histological type (Table 1).
Univariate survival analysis reveals an association between worse prognosis and advanced tumour stage, suboptimal debulking surgery with postoperative residual tumour, high histological grade, increased patient’s age and elevated pre-therapeutic GGT serum levels. Results of univariate survival analyses are shown in Table 2. Figure 1 provides Kaplan–Meier curve for un-pooled GGT risk groups (A vs B vs C vs D) with respect to overall survival (P<0.001). In multivariable analysis elevated pre-therapeutic GGT serum levels are confirmed to be an independent prognostic factor for worse overall survival in patients with EOC (Table 2). There is no evidence of a non-linear effect of GGT on survival as evidenced by the multivariable fractional polynomial approach. The adjusted effect of continuous GGT on survival is highly predictive (HR=1.056 per U l−1, 95% CI: 1.02–1.09, P=0.002). The cross-validated c-index of the multivariable Cox regression model is 0.777, indicating adequate discrimination of survivors from non-survivors across the entire range of follow-up time.
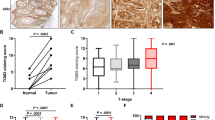
To further characterise the role of GGT serum levels in tumour progression and prognosis, the association between systemic GGT serum levels and local GGT expression in the tumour tissue by IHC has been assessed. Elevated pre-therapeutic GGT serum levels are associated with elevated local GGT expression in the epithelial tumour tissue (P=0.03, Figure 2).
Representative immunohistochemical staining of GGT in two different EOC samples showing ( A ) GGT negative expression and ( B ) GGT strong expression. Pictures were taken using TissueFAXS (TissueGnostics, Vienna, Austria; optical magnification x200). A full colour version of this figure is available at the British Journal of Cancer journal online.
Discussion
In the present multicenter study, high GGT serum levels were independently associated with worse prognosis in patients with EOC. We aimed to further characterise the role of GGT in patients with EOC and observed that patients with EOC had higher GGT serum levels than patients with BTO. In patients with EOC, elevated pre-therapeutic GGT serum levels were associated with higher local GGT expression in the tumour tissue and advanced tumour stage.
Our finding that pre-therapeutic GGT serum level is an independent prognostic factor in patients with EOC is in line with previous studies in cervical and endometrial cancer (Polterauer et al, 2011; Seebacher et al, 2012). We did not aim to identify a prognostic cutoff value, as GGT serum levels seem to correlate with overall survival in a linear way (Polterauer et al, 2011). This was confirmed by multivariable fractional polynomial analysis revealing a continuous negative effect of increasing GGT serum levels on overall survival, HR: 1.056 (1.02–1.09) per GGT U l−1. Thus, we stratified the patients in four previously established and clinically relevant risk groups with respect to their pre-therapeutic GGT serum level (Strasak et al, 2008a; Polterauer et al, 2011; Seebacher et al, 2012). Patients in group D (highly elevated GGT) had significantly shorter overall survival times compared to the reference group (Table 2).
In the present analysis, pre-therapeutic GGT serum levels provided independent prognostic information in addition to established prognostic parameters, such as FIGO stage, histological grade, postoperative residual disease and patient’s age. Clinically, it seems plausible that GGT, a marker of oxidative stress, provides additional prognostic information to tumour stage. By revealing the amount of pathologic oxidative stress in cancer patients, GGT most likely reflects the extent of malignant cell transformation and cell turnover caused not only by the extent of tumour load, but also by the tumour’s aggressiveness. In our study, GGT serum levels were higher in patients with EOC than in patients with BTO, supporting the association between GGT and tumour aggressiveness. Moreover, in patients with EOC, higher GGT serum levels were associated with advanced tumour stage reflecting the association between GGT and tumour load. Both findings are in line with large studies in patients with premalignant cervical lesions and cervical cancer (Strasak et al, 2010; Polterauer et al, 2011).
In a next step, we aimed to identify the association between systemic GGT serum levels and local GGT tissue expression in the tumour, ascertained by immunohistochemistry. The finding that systemic GGT levels were significantly associated with local GGT expression in the tumour tissue of patients with EOC is biologically particularly interesting. It can be speculated that the increase of GGT serum levels directly results of release of GGT from cancer cells. In vitro studies demonstrated that different cancer cell lines release soluble GGT, subsequently resulting in increased GGT serum levels (Yao et al, 1998; Franzini et al, 2009). This suggests a neoplastic origin of elevated GGT serum levels. Moreover it has been hypothesised that GGT not only reflects malignant cell transformation but also triggers carcinogenesis. Pro-oxidant reactions produced by GGT may contribute to the ‘persistent oxidative stress’ described as a factor in genomic instability and carcinogenesis (Stark et al, 1988, 1994; Corti et al, 2010). Next to the promotion of carcinogenesis, GGT seems to induce mutagenesis, modulate cellular proliferation and apoptotic balance by its redox-sensitive function (Dominici et al, 1999; Pompella et al, 2007; Corti et al, 2009). Therefore, it can be reasonably speculated that tissue expression of GGT in EOC might have a direct effect on tumour progression and aggressive tumour behaviour. In this case GGT might even be interesting as a potential novel therapeutic target, by using it as a factor for targeting nitric oxide to tumour tissue or for the activation of gamma-glutamyl pro-drugs. Recently, a novel class of uncompetitive inhibitors of GGT, less toxic than glutamine analogues, has been described (Daubeuf et al, 2001; Corti et al, 2010).
Of note, our study has several limitations. Some parameters were not available in all patients and due to the retrospective design of our study it was impossible to retrieve all missing information. Another potential bias could have been patients’ co-morbidities influencing GGT serum levels. However, we estimate this risk as minimal, as all patients had a pre-therapeutic assessment by a consultant in internal medicine, including a complete laboratory work-up. Patients with identified co-morbidities known to be associated with elevated GGT (i.e. hepato- biliary tract-, pancreatic- and heart disease or alcohol abuse) were not included in the present study. Information about patient’s medication was only partially available. Therefore we were not able to stratify patients according to known drugs that might alter GGT serum levels (Wannamethee et al, 1995; Santos et al, 2003; Mabile et al, 2003). Nonetheless, given the large number of patients, it seems unlikely that our results were significantly biased by patients with undetected disease or unavailable medication information.
Pre-therapeutic GGT serum level is an independent prognostic marker in patients with epithelial ovarian cancer. Gamma-glutamyltransferase serum levels adequately reflect GGT expression in EOC tumour tissue. Whether GGT is actively involved in carcinogenesis and tumour progression or only reflects the extent of cancerous metabolism remains unclear. Further research is needed to address the exact biological mechanisms linking GGT to cancer progression and prognosis.
Change history
06 August 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Benedet JL, Bender H, Jones H 3rd, Ngan HY, Pecorelli S (2000) FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet 70: 209–2262.
Corti A, Duarte TL, Giommarelli C, De Tata V, Paolicchi A, Jones GD, Pompella A (2009) Membrane gamma-glutamyl transferase activity promotes iron-dependent oxidative DNA damage in melanoma cells. Mutat Res 669: 112–121.
Corti A, Franzini M, Paolicchi A, Pompella A (2010) Gamma-glutamyltransferase of cancer cells at the crossroads of tumour progression, drug resistance and drug targeting. Anticancer Res 30: 1169–1181.
Daubeuf S, Accaoui MJ, Pettersen I, Huseby NE, Visvikis A, Galteau MM (2001) Differential regulation of gamma-glutamyltransferase mRNAs in four human tumour cell lines. Biochim Biophys Acta 1568: 67–73.
Dominici S, Valentini M, Maellaro E, Del Bello B, Paolicchi A, Lorenzini E, Tongiani R, Comporti M, Pompella A (1999) Redox modulation of cell surface protein thiols in U937 lymphoma cells: the role of gamma-glutamyl transpeptidase-dependent H2O2 production and S-thiolation. Free Radic Biol Med 27: 623–635.
Fentiman IS, Allen DS (2010) gamma-Glutamyl transferase and breast cancer risk. Br J Cancer 103: 90–93.
Franzini M, Corti A, Lorenzini E, Paolicchi A, Pompella A, De Cesare M, Perego P, Gatti L, Leone R, Apostoli P, Zunino F (2006) Modulation of cell growth and cisplatin sensitivity by membrane. Gamma -glutamyltransferase in melanoma cells. Eur J Cancer 42: 2623–2630.
Franzini M, Corti A, Fornaciari I, Balderi M, Torracca F, Lorenzini E, Baggiani A, Pompella A, Emdin M, Paolicchi A (2009) Cultured human cells release soluble gamma-glutamyltransferase complexes corresponding to the plasma b-GGT. Biomarkers 14: 486–492.
Hanigan MH (1995) Expression of gamma-glutamyl transpeptidase provides tumour cells with a selective growth advantage at physiologic concentrations of cyst(e)ine. Carcinogenesis 16: 181–185.
Hanigan MH, Gallagher BC, Townsend DM, Gabarra V (1999) Gamma-glutamyl transpeptidase accelerates tumour growth and increases the resistance of tumors to cisplatin in vivo. Carcinogenesis 20: 553–559.
Kazemi-Shirazi L, Endler G, Winkler S, Schickbauer T, Wagner O, Marsik C (2007) g Glutamyltransferase and long-term survival: is it just the liver? Clin Chem 53: 940–946.
Mabile L, Ruidavets JB, Fauvel J, Perret B, Ferrières J (2003) Differential levels of gamma-glutamyl transferase activity and apolipoprotein CIII in men on either statin or fibrate therapy. Diabetes Care 26: 1652–1653.
Polterauer S, Hofstetter G, Grimm C, Rahhal J, Mailath-Pokorny M, Kohl M, Concin N, Tempfer C, Marth C, Reinthaller A (2011) Relevance of gamma-glutamyltransferase – a marker for apoptotic balance – in predicting tumor stage and prognosis in cervical cancer. Gynecol Oncol 122: 590–594.
Pompella A, De Tata V, Paolicchi A, Zunino F (2006) Expression of -glutamyltransferase in cancer cells and its significance in drug resistance. Biochem Pharmacol 71: 231–238.
Pompella A, Corti A, Paolicchi A, Giommarelli C, Zunino F (2007) Gamma-glutamyltransferase, redox regulation and cancer drug resistance. Curr Opin Pharmacol 7: 360–366.
Royston P, Altman DG (1994) Regression using fractional polynominals of continuos covariates: Parsimonious parametric modeling. Apl Statistics 43: 429–467.
Santos VN, Lanzoni VP, Szejnfeld J, Shigueoka D, Parise ER (2003) A randomized double-blind study of the short-time treatment of obese patients with nonalcoholic fatty liver disease with ursodeoxycholic acid. Braz J Med Biol Res 36: 723–729.
Seebacher V, Polterauer S, Grimm C, Rahhal J, Hofstetter G, Bauer EM, Husslein H, Leipold H, Marth C, Reinthaller A, Concin N (2012) Prognostic significance of gamma-glutamyltransferase in patients with endometrial cancer: a multi-centre trial. Br J Cancer 106: 1551–1555.
Stark AA, Zeiger E, Pagano DA (1988) Glutathione mutagenesis in Salmonella typhimurium is a gamma-glutamyltranspeptidase-enhanced process involving active oxygen species. Carcinogenesis 9: 771–777.
Stark AA, Pagano DA, Glass G, Kamin-Belsky N, Zeiger E (1994) The effects of antioxidants and enzymes involved in glutathione metabolism on mutagenesis by glutathione and L-cysteine. Mutat Res 308: 215–222.
Strasak A, Pfeiffer R, Klenk J, Hilbe W, Oberaigner W, Gregory M, Concin H, Diem G, Pfeiffer KP, Ruttmann E, Ulmer H Vorarlberg Health Monitoring and Promotion Program Study Group (2008a) Prospective study of the association of gamma-glutamyltransferase with cancer incidence in women. Int J Cancer 123: 1902–1906.
Strasak AM, Rapp K, Brant LJ, Hilbe W, Gregory M, Oberaigner W, Ruttmann E, Concin H, Diem G, Pfeiffer KP, Ulmer H and the VHM & PP Study Group (2008b) Association of gamma-glutamyltransferase and the risk of cancer incidence in men: a prospective study. Cancer Res 68: 3970–3977.
Strasak AM, Goebel G, Concin H, Pfeiffer RM, Brant LJ, Nagel G, Oberaigner W, Concin N, Diem G, Ruttmann E, Gruber-Moesenbacher U, Offner F, Pompella A, Pfeiffer KP, Ulmer H VHM & PP Study Group (2010) Prospective study of the association of serum gamma-glutamyltransferase with cervical intraepithelial neoplasia III and cervical cancer. Cancer Res 70: 3586–3593.
Uno H, Cai T, Pencina MJ, Agostino RB, Wie LJ (2011) On the c-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med 30: 1105–1117.
Van Hemelrijck M, Jassem W, Walldius G, Fentiman IS, Hammar N, Lambe M, Garmo H, Jungner I, Holmberg L (2011) Gamm-glutamyltransferase and risk of cancer in a cohort of 545,460 persons – the Swedish AMORIS study. Eur J Cancer 47: 2033–2041.
Wannamethee G, Ebrahim S, Shaper AG (1995) Gamma-glutamyltransferase: determinants and association with mortality from ischemic heart disease and all causes. Am J Epidemiol 142: 699–708.
Whitfield JB (2001) Glutamyl transferase. Crit Rev Clin Lab Sci 38: 263–355.
Yao DF, Huang ZW, Chen SZ, Huang JF, Lu JX, Xiao MB, Meng XY (1998) Diagnosis of hepatocellular carcinoma by quantitative detection of hepatoma-specific bands of serum gamma-glutamyltransferase. Am J Clin Pathol 110: 743–749.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Grimm, C., Hofstetter, G., Aust, S. et al. Association of gamma-glutamyltransferase with severity of disease at diagnosis and prognosis of ovarian cancer. Br J Cancer 109, 610–614 (2013). https://doi.org/10.1038/bjc.2013.323
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.323
Keywords
This article is cited by
-
MicroRNA-142-3p promotes renal cell carcinoma progression by targeting RhoBTB3 to regulate HIF-1 signaling and GGT/GSH pathways
Scientific Reports (2023)
-
Does gamma-glutamyltransferase correlate with liver tumor burden in neuroendocrine tumors?
Endocrine (2023)
-
Metabolomic profile of prostate cancer-specific survival among 1812 Finnish men
BMC Medicine (2022)
-
Liver enzymes and risk of ocular motor cranial nerve palsy: a nationwide population-based study
Neurological Sciences (2022)
-
Predictive clinical factors of chronic peripheral neuropathy induced by oxaliplatin
Supportive Care in Cancer (2020)