Abstract
Background:
Bcl-xL has an important role in the control of cell death through its inhibition of apoptosis. The aim of this study was to investigate the clinicopathological significance of Bcl-xL in upper urinary tract urothelial carcinoma (UTUC) and the therapeutic effect of targeting Bcl-xL protein in urothelial carcinoma (UC) cells.
Methods:
We evaluated the immunohistochemical expression of Bcl-xL in 175 UTUC patients to determine the clinical role of Bcl-xL expression in clinical outcome. We used bafilomycin A1 (BMA) as a specific inhibitor of Bcl-xL to examine the biological effects in UC cells in vitro and in vivo.
Results:
Immunohistochemical analysis of Bcl-xL expression revealed that patients with a high Bcl-xL score had a significantly lower 5-year cancer-specific survival (CSS) rate (53.2%) than those with a low Bcl-xL score (77.2%) (P=0.0011). Multivariate analysis indicated that a high Bcl-xL score was an independent prognostic factor of CSS (P=0.023). BMA inhibited UMUC-3 cell proliferation in vitro by induction of apoptosis. Treatment with BMA significantly inhibited tumour growth in UMUC-3 tumours in this mouse xenograft model accompanied by an elevated apoptosis induction.
Conclusion:
Bcl-xL appears to be a significant molecular marker for the prognosis of UTUCs. Targeting Bcl-xL may be a promising therapeutic strategy for patients with UC.
Similar content being viewed by others
Main
Bladder cancer is the most common neoplasm occurring along the urothelium. By contrast, upper urinary tract urothelial carcinomas (UTUCs) are relatively rare, accounting for approximately 10% of all renal tumours and approximately 5% of all urothelial carcinomas (UCs; Oosterlinck, 2007). Radical nephroureterectomy (RNU) with bladder cuff excision is the standard procedure for UTUC. As UTUCs are a relatively rare type of tumour, there are few molecular markers known to predict the disease prognosis in UTUC patients (Hall et al, 1998; Nakanishi et al, 1998; Fromont et al, 2005; Miyata et al, 2005; Kashibuchi et al, 2006; Catto et al, 2007; Jeong et al, 2009; Kosaka et al, 2010).
Although 20–30% of bladder cancers have the invasive stage at the initial presentation, approximately 60% of UTUC are diagnosed at the invasive stage (Hall et al, 1998; Kirkali et al, 2005). Even if RNU was performed for UTUC patients, >20% of UTUC patients experienced extravesical disease recurrence or distant metastasis during follow-up (Rink et al, 2012). Therefore, it is important to predict which UTUC patients are most likely to have recurrence and poor survival and to conduct follow-up in order to classify their risks.
Bcl-xL is one of the Bcl-2 family proteins and has an important role in controlling cell death and in the response of cancer cells to chemotherapeutic drugs or radiation therapy (Cotter, 2009). Bcl-xL molecules may inhibit apoptosis by maintaining the permeabilisation status or stabilisation of the outer mitochondrial membrane (Boise et al, 1993). Targeting the anti-apoptotic Bcl-2 family proteins can induce apoptosis and thus overcome drug resistance to cancer chemotherapy (Kang and Reynolds, 2009). Although Bcl-2 was not overexpressed frequently in UC of urinary bladder (Kirsh et al, 1998), Bcl-xL is expressed at a high frequency (Lebedeva et al, 2001; Korkolopoulou et al, 2002), therefore, it might become the target of treatment in UC. Several studies have evaluated the prognostic role of Bcl-xL protein expression in bladder carcinoma, especially for superficial bladder carcinoma (Gabriel et al, 2008; Bolenz et al, 2009). However, the status of Bcl-xL expression in UTUC tissues and its prognostic significance are unclear.
Vacuolar H+-ATPase (V-ATPase) is the specific proton pump of cells and its inhibitors have been shown to induce apoptosis of cancer cell lines (Ohta et al, 1998; Nakashima et al, 2003; Morimura et al, 2008). More recently, Bcl-xL protein has been shown to be the target for V-ATPase inhibitors such as bafilomycin A1 (BMA) in cancer cells (Sasazawa et al, 2009). Therefore, BMA may regulate the function of Bcl-xL protein in cancer cells and exhibit an anticancer effect. Whether targeting therapy for Bcl-xL proteins, such as BMA, could induce apoptosis and has an anti-tumour effect in UC cells have never been evaluated.
Therefore, in the present study, we evaluated (i) the association between the tumour characteristics of 175 cases of UTUC and the expression of Bcl-xL by immunohistochemistry with slides re-reviewed by genitourinary pathologists to determine the clinical role of Bcl-xL expression in survival in UTUC patients, and (ii) examined whether targeting therapy for Bcl-xL using BMA could induce apoptosis and have a therapeutic effect on UC cells in vitro and in vivo.
Materials and methods
Immunohistochemical evaluation of Bcl-xL in UTUC human samples
A total of 201 patients had undergone surgical treatment for UTUC at Keio University Hospital from 1999 to 2007. This study design was approved by the ethics review board of Keio University and the medical records of patients were retrospectively reviewed. The median follow-up period was 35.0 months and mean patient age was 66.2 years old (range, 36–89). RNU was performed in 196 patients and partial ureterectomy was performed in 5 patients. Local lymph node dissection was performed in patients with a suspected adenopathy observed pre- or intraoperatively. Extended lymph node dissection was not routinely performed. We identified a total of 175 patients with pathological Ta-T4N0M0 tumours (pTa-4N0M0) in our study population (pTa-pT1: 57 patients, pT2: 37 patients, pT3: 77 patients, pT4: 4 patients). Patients with distant metastasis at diagnosis or positive lymph node involvement were excluded from the analysis. Cisplatin-based adjuvant chemotherapy regimens were administered to 26 patients (14.9%). The patients were followed postoperatively with urinary cytology every 3 months for 2 years and every 6 months thereafter. Computed tomography or magnetic resonance imaging as well as cystoscopy were performed every 6 months for 5 years and annually thereafter.
All tumour specimens were fixed in 10% formalin and embedded in paraffin, and all slides were re-reviewed by uropathologists. Tumours were staged according to the American Joint Committee on Cancer–Union Internationale Contre le Cancer TNM classification. Tumour grading was assessed according to the 1998 WHO/International Society of Urologic Pathology consensus classification (Epstein et al, 1998). Lymphovascular invasion (LVI) was defined as the presence of tumour cells within an endothelium-lined space without underlying muscular walls.
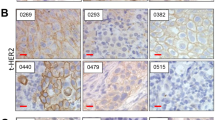
Sections (4 μm) of formalin-fixed and paraffin-embedded material were analysed. The sections were deparaffinised in xylene and rehydrated in graded alcohols and distilled water. After antigen retrieval with Target Retrieval Solution (pH 9.0) (Dako, Glostrup, Denmark), endogenous peroxidase activity was blocked with 1% hydrogen peroxide for 30 min followed by washing with distilled water. To bind nonspecific antigens, the sections were incubated with 5% skim milk for 15 min. The sections were incubated with a primary Bcl-xL mouse monoclonal antibody (1 : 100 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at room temperature for 1 h. After washing with PBS, they were incubated with secondary antibodies for 30 min. The antibody was detected using the avidin–biotin complex peroxidase method. Color was developed with 3,3′-diaminobenzamine tetrahydrochloride in 50 mmol l−1 Tris-HCl (pH 7.5) containing 0.005% hydrogen peroxide. The specifity of the antibody was examined using blocking peptides (Santa Cruz Biotechnology) to inhibit adsorption for Bcl-xL antibody, resulting in a significantly reduced staining reaction as compared with the staining with the primary antibody (Figures 1E and F). The sections were counterstained with hematoxylin.
Immunostaining of Bcl-xL protein expression in upper urinary tract carcinoma tumour tissue. (A) Bcl-xL score 0 points (negative staining), (B) Bcl-xL score 4 points, (C) Bcl-xL score 8 points, and (D) Bcl-xL score 12 points. (E) Immunostaining with primary antibody for Bcl-xL, (F) addition of blocking peptide for Bcl-xL to primary antibody for Bcl-xL, resulting in a significantly reduced staining reaction (bars=100 μm).
The results of Bcl-xL immunostaining were scored as described previously (Krajewska et al, 1996). To evaluate Bcl-xL staining, cancer cells with positive staining in the cytoplasm were counted in at least 10 representative fields selected randomly by a uro-pathologist who was unaware of the clinicopathological data and clinical outcomes of the patients. The percentage of positive tumour cells was graded as follows: 0, none; 1, 1–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100%. The intensity of the tumour cells was rated as follows: 0, none; 1, weak; 2, moderate; and 3, intense. The score was calculated by multiplication of the grading score of the percentage of positive tumour cells and that of intensity, and is expressed in the range of 0–12. To evaluate the Bcl-xL expression and the prognostic value of UTUC patients, we divided the Bcl-xL score into two groups according to the intensity of Bcl-xL expression. A high Bcl-xL score and a low Bcl-xL score was defined as 9–12 and 0–8, respectively.
Cell culture and materials
The UMUC-3 UC cell line was routinely maintained in RPMI-1640 (Invitrogen, Tokyo, Japan) with 10% fetal bovine serum (FBS) at 37 °C in a humidified 5% CO2 atmosphere. UMUC-3 was obtained from the American Type Culture Collection. BMA was purchased from Wako Pure Chemical Industries (Osaka, Japan). BMA was dissolved in dimethyl sulphoxide (DMSO) and diluted to appropriate concentrations with culture medium.
Cell growth assay
UMUC-3 cells were plated in 96-well plates, allowed to adhere for 24 h, and then treated with various concentrations of BMA. Cells treated with the same concentrations of DMSO served as controls. At the end of the incubation period, cytotoxicity was determined using WST-1; 4-[3-(4-lodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1, 3-benzene disulphonate (Takara Bio Inc., Shiga, Japan). The absorbance value of each well was determined at 450 nm with a 650 nm reference beam by a microplate reader (Bio-Rad Laboratories, Inc., Tokyo, Japan). The BMA dose used in the cell viability assay was the same as that of previous reports in gastric cancer and pancreatic cancer (Ohta et al, 1998; Nakashima et al, 2003). These experiments were repeated on different days when new cells had grown.
Western blot analysis
Whole-cell extracts were obtained using radioimmunoprecipitation assay buffer (50 mmol l−1 Tris-HCL (pH 7.5), 150 mmol l−1 NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS) containing protease inhibitors. For western blot analysis, 50 μg of total protein for Bcl-xL, cytochrome c, and caspase-3 (Santa Cruz Biotechnology) from each sample were loaded on 12.5% SDS–polyacrylamide gels. Immunoblotting was also performed according to a standard method. Proteins were transferred onto a polyvinylidene difluoride membrane in blocking solution (5% nonfat dry milk in TBS containing 0.1% Tween 20). Primary antibodies for Bcl-xL (1 : 200 dilution), cytochrome c (1 : 200 dilution), and caspase-3 (1 : 200 dilution) were used for the study. After washing, the membranes were incubated for 1.5 h at room temperature linked with peroxidase secondary antibody (Dako, Glostrup, Denmark), and then the proteins were visualised on X-ray film using an electrochemiluminescence western blotting detection kit (PerkinElmer Life Science, Waltham, MA, USA).
Flow cytometric analysis for the detection of apoptosis
Flow cytometric analysis was performed using TUNEL assay for detecting apoptosis and BrdU assay for cell cycle analysis. Briefly, cells (1 × 106) were plated in 100 mm dishes and allowed to attach overnight. They were then treated with 5 nM of BMA for 12 h. Next, the cells were harvested and fixed in 70% ethanol at 4 °C overnight, resuspended in PBS containing 0.05 mg ml−1 RNase A (Sigma Chemical, St. Louis, MO, USA), and incubated at room temperature for 30 min. After washing, the cells were stained with FITC-labeled BrdU (BD Biosciences, Franklin Lakes, NJ, USA) and propidium iodide and analysed by flow cytometry (Beckman Coulter, Fullerton, CA, USA). TUNEL assay was performed using ApopTag Kits (Sigma Chemical) according to the manufacturer’s protocol, and apoptosis was detected by flow cytometry (Beckman Coulter).
Small interfering RNA (siRNA)
Bcl-xL expression was transiently downregulated using the following predesigned duplex siRNA directed against Bcl-xL (siBcl-xL; Ambion, Carlsbad, CA, USA). The sense sequences of siRNA for Bcl-xL were as follows: siBcl-xLA, 5′-AUACUUUUGUGGAACUCUAtt-3′; and siBcl-xLB, 5′-GGAACUCUAUGGGAACAAUtt-3′. UMUC-3 cells were cultured in antibiotic-free medium overnight at 37 °C in 5% CO2 and then cells were transiently transfected with 20 nmol of siBcl-xLA and siBcl-xLB using Lipofectamine 2000 (Invitrogen Co., Tokyo, Japan). After 4 h, siRNA was removed by replacing the culture medium with fresh RPMI 1640 containing 10% FBS, and cells were cultured for additional 48–72 h. A mock-transfection control was prepared using the transfection reagent only.
Treatment in vivo
BALB/c mice, 6 weeks of age with an average body weight of 20 g, were purchased from Sankyo Laboratory Service (Tokyo, Japan). Mice were housed under specific pathogen-free conditions. All of the procedures involving animals and their care in this study were approved by the Animal Care Committee of Keio University in accordance with institutional and the Japanese government guidelines for animal experiments. All mice were inoculated subcutaneously (s.c.) in the flank with 100 μl (1 × 106 cells) of UMUC-3 cells. When the mice had developed palpable tumours, they were randomly assigned to two groups, each consisting of eight animals. An animal was then given an intraperitoneal injection of 1 mg kg−1 of BMA or vehicle control every 4 days for 24 consecutive days. The animals were carefully monitored, and the tumour was measured every 4 days. The mice were killed, and the tumours were collected 28 days after treatment. The tumour volume (V) was calculated according to the formula V=AB2/2, where A is the greatest diameter and B is the diameter at the point perpendicular to A.
For histological examinations, tumour specimens were fixed in 4% paraformaldehyde, processed by routine methods, embedded in paraffin wax, and sectioned at 4 μm. Immunohistochemical staining with TUNEL assay was carried out on sections of formalin-fixed paraffin wax-embedded tissue. Apoptosis was measured using a commercially available in situ apoptosis detection kit (Takara Bio Inc., Shiga, Japan). Visualisation of the immunoreaction was performed with 0.06% 3, 3′-diaminobenzidine (DAB; Sigma Chemical). A dark accumulation of DAB in the nuclei indicated a positive reaction for TUNEL.
Statistical analysis
The differences between the Bcl-xL score and clinicopathological variables were analysed using the Mann–Whitney U test. Cancer-specific survival (CSS) calculated by the Kaplan–Meier method was evaluated using the log-rank test. We used Cox’s proportional hazards regression analysis to assess the prognostic indicators that included age, gender, tumour stage, grade, tumour location, LVI, and Bcl-xL score for CSS and bladder recurrence-free survival. The difference between the two groups in in vitro study and in the animal model was assessed with the Mann–Whitney U test.The level of statistical significance was set at P<0.05. These analyses were performed with the SPSS Version 16.0 statistical software package (SPSS Corporation, Chicago, IL, USA).
Results
Clinical role of Bcl-xL in UTUC human samples
Bcl-xL expression in UTUC
Immunohistochemical evaluation was performed to detect the level of Bcl-xL protein expression in UTUC tissue samples from 175 cases. Representative images for Bcl-xL scores of 0, 4, 8, and 12 are shown in Figures 1A, B, respectively. Positive staining of Bcl-xL protein was mainly located in the cytoplasm of tumour cells in UTUC tissues. Overall, 164 patients (93.7%) had positive immunostaining of Bcl-xL protein and 11 tumour tissues showed negative staining (score 0). The mean±s.d. of the Bcl-xL score was 6.50±3.68 in all human samples. The Bcl-xL score was 0–4 in 70 tumour tissues, 5–8 in 56, and 9–12 in 49. The Bcl-xL score for high tumour stages ⩾pT2 was 7.36±3.54, which was significantly higher than that for low tumour stages P<0.001) (Table 1). Patients with a high tumour grade had a higher Bcl-xL score (7.04±3.74) compared with patients with a low tumour grade (5.56±3.40, P=0.007). Positive LVI was more associated with a higher Bcl-xL score (7.89±3.43) than negative LVI (5.84±3.62, P=0.001). However, there were no significant differences in age (<65 or ⩾65 years: P=0.085) or tumour location (pelvis or ureter: P=0.993) with respect to the Bcl-xL score.
Prognostic significance of Bcl-xL score in CSS of UTUC patients
Kaplan–Meier curve analysis demonstrated that patients with a high Bcl-xL score (Bcl-xL score ⩾9) had a significantly lower CSS than those in the low Bcl-xL score group (Bcl-xL score <9) (P=0.0011) (Figure 2A). The 5-year CSS in the high Bcl-xL score group and low Bcl-xL score group was 53.2% and 77.2%, respectively.
Kaplan–Meier curves of cancer-specific survival (CSS) or bladder recurrence according to the expression level of Bcl-xL score. (A) CSS curve in overall patients, (B) bladder recurrence-free survival curve in overall patients, (C) CSS in patients with ⩾pT2 tumour stage, (D) CSS in patients with <pT2 tumour stage, (E) CSS in high-grade tumour patients, (F) CSS in low-grade tumour patients, (G) CSS in lymphovascular invasion (LVI)-positive patients, and (H) CSS in LVI-negative patients.
In a subgroup of patients with pT2 or greater UTUC tumour (n=118, Figure 2C), there were significant differences in CSS between the high and low Bcl-xL score groups (P=0.0227). The 5-year CSS in ⩾pT2 patients in the high Bcl-xL score group and low Bcl-xL score group was 48.7% and 67.4%, respectively. In a subgroup of patients with a tumour n=57, Figure 2D) and low-grade tumour patients (n=64, Figure 2F), there were no significant differences in CSS between the two Bcl-xL groups. In a subgroup of patients with a high-grade UTUC tumour (n=111, Figure 2E), there were significant differences in CSS between the high and low Bcl-xL score groups (P=0.0327). The 5-year CSS in high-grade patients with a high Bcl-xL score and a low Bcl-xL score was 45.0% and 64.9%, respectively. Furthermore, in a subgroup of patients who were LVI positive (n=56), a significant difference in CSS between the high and low Bcl-xL score groups was observed (P=0.0074; Figure 2G). The 5-year CSS in LVI-positive patients with a high Bcl-xL score and a low Bcl-xL score was 20.1% and 51.5%, respectively. By contrast, no significant difference in CSS was observed between the two Bcl-xL groups in patients who were LVI negative (n=119, Figure 2H).
Bladder recurrence was observed in 72 patients (41.1%), and the median bladder recurrence-free period was 9.5 months during follow-up after RNU. Kaplan–Meier curve analysis demonstrated that there was no significant difference between the high and low Bcl-xL score groups with respect to the bladder recurrence-free rate (P=0.3276) (Figure 2B). Furthermore, in a subgroup analysis according to tumour grade, pT stage, or LVI status, no differences in the bladder recurrence rate between the high and low Bcl-xL score groups were observed (data not shown).
Table 2 shows the results of univariate and multivariate Cox regression analyses with regard to the CSS and bladder recurrence-free survival. Univariate analysis revealed that tumour stage, tumour grade, presence of LVI, and Bcl-xL score were significantly associated with CSS. Multivariate analysis revealed that the presence of LVI and a high Bcl-xL score were independent prognostic factors of CSS (P<0.001 and P=0.023, respectively). No clinicopathological feature or Bcl-xL score was independently associated with bladder recurrence-free survival.
Therapeutic efficacy of targeting therapy for Bcl-xL using BMA in in vitro study
Cell viability assay of UMUC-3 cells treated by BMA
On the basis of the prognostic value of Bcl-xL expression in UTUC patients, we investigated whether targeting therapy for Bcl-xL would have a therapeutic effect on UC cells by using BMA, which specifically inhibits Bcl-xL expression. Almost all UC cell lines tested expressed Bcl-xL protein. In those cell lines, UMUC-3 cells showed one of the highest expression levels of Bcl-xL (Figure 3A). Therefore we decided to use UMUC-3 cells for this study.
Targeting therapy for Bcl-xL in vitro study using BMA. (A) Western blot analyses of Bcl-xL expression in various bladder cancer cell lines. The expression level of bladder cancer cell lines (5637, TCCSUP, RT4, UMUC-3, and T24) with western blot analysis. (B, C) Cell growth inhibitory effects of BMA in UMUC-3 cells. The cells were treated with (B) 48-h and (C) 72-h exposure to 5 nM or 10 nM BMA and cell viability was measured by WST-1 assay. Cells treated with the same concentrations of DMSO served as controls. *P<0.01 compared with control. (D–G) Detection of apoptosis by 5 nM BMA flow cytometric analyses were performed by TUNEL assay and BrdU assay to detect apoptotic cells in (D, F) vehicle control and (E, G) 5 nM BMA treatment, respectively. Upper left quadrants of each graph represent the apoptotic cell populations. Cells treated with the same concentrations of DMSO served as controls. (H) Western blot analyses on Bcl-xL, cytochrome c, and caspase-3 protein. Western blot analysis was performed using antibodies specific for Bcl-xL, cytochrome c, and caspase-3. Protein was prepared after exposure to various concentrations of BMA. Cells treated with the same concentrations of DMSO served as controls. (I) siRNA for Bcl-xL in UMUC-3 cells. Western blotting for Bcl-xL protein, 48 h after transfection with the concentration of 20 nM siBcl-xLA and siBcl-xLB, respectively. (J) Cell viability assay for UMUC-3 cells with 20 nM siBcl-xLA and siBcl-xLB 72 h after transfection was measured by WST-1 assay, *P<0.05 compared with control.
UMUC-3 cells were grown in the absence or presence of various concentrations of BMA for 48 and 72 h (Figures 3B and C). In UMUC-3 cells, the mean cell viability following treatment with 5 and 10 nM BMA for 48 h was 60.0±4.5% (P<0.01) and 47.5±4.8% (P<0.01), respectively, compared with vehicle control. Similar results were observed 72 h after BMA exposure; the mean cell viability following treatment with 5 and 10 nM BMA was 41.2±4.1% (P<0.01) and 29.9±3.8% (P<0.01), respectively, compared with vehicle control.
Apoptosis and cell-cycle assay
The TUNEL and cell-cycle assays were performed to detect apoptotic cells and cell-cycle distribution after 5 nM BMA treatment. Higher levels of apoptotic cells were observed after 5 nM BMA treatment (36.4% of apoptotic cells in BMA treatment, Figure 3E vs 0.17% of those in vehicle control, Figure 3D). In the BrdU assay, cancer cells accounted for 57.6% of cells (Figure 3G) in the sub-G1 phase of the cell cycle compared with 4.4% for control cells (Figure 3F).
Effect of BMA on Bcl-xL and apoptotic-related protein expression
Western blot analysis was performed to confirm whether BMA had an effect on Bcl-xL expression and the related apoptotic protein (Figure 3H). Bcl-xL protein was expressed in untreated control UMUC-3 cells. A dose-dependent decrease in Bcl-xL protein expression was observed after 5 and 10 nM BMA treatment. Caspase-3 expression and cytochrome c protein expression were increased in the BMA treatment group compared with the controls.
Effect of siRNA for Bcl-xL in UMUC-3 cells
UMUC-3 cells were transiently transfected with 20 nM of siBcl-xLA and siBcl-xLB. To evaluate the inhibition of Bcl-xL protein, we performed western blot analysis for cells treated with these siRNAs. siBcl-xLA and siBcl-xLB demonstrated significant reduction of Bcl-xL expression compared with control. Figure 3I shows the results of western blotting for Bcl-xL protein, 48 h after transfection with 20 nM siBcl-xLA and 20 nM siBcl-xLB. Cell viability assay using WST-1 reagent revealed significant inhibition of cell viability in UMUC-3 cells 72 h after being treated with 20 nM of these siRNAs. The inhibition of cell viability of UMUC-3 cells transfected with siBcl-xLA and siBcl-xLB was 80.8±3.0% (P<0.05) and 81.9±3.7% (P<0.05), respectively, compared with control after 72 h (Figure 3J).
Anti-tumour effect of BMA in UC xenograft model
Based on the in vitro findings, we tested the anti-tumour effects of BMA in an in vivo study using a UMUC-3 subcutaneous tumour model. The results of this in vivo study demonstrated a significant decrease in tumour growth in BMA-treated mice compared with control mice on days 16, 20, and 24 after the start of BMA treatment (Figure 4A). The mean±s.d. of tumour volume was 1300±260 mm3 in the BMA-treated group and 2400±510 mm3 in the control group on day 20 after starting the treatment. TUNEL staining of tumours was performed to evaluate whether apoptosis was induced in BMA-treated xenograft tumours. A large number of tumour cells showed signs of apoptosis after BMA treatment, as confirmed by TUNEL staining (Figure 4E).
Efficacy of BMA on tumour growth in xenograft UMUC-3 tumour model. (A) UMUC-3 cells (1 × 106) were implanted subcutaneously into the flank of nude mice. When an animal developed a palpable tumour, it was given an intraperitoneal injection of 1 mg kg−1 of BMA or vehicle control every 4 days for 24 consecutive days. BMA was injected a total of six times in the treatment group. Tumour volume was monitored every 4 days. *Statistically significant difference compared with vehicle controls, P<0.01. (B–E) Histopathogical evaluation in BMA-treated UMUC-3 tumours. Hematoxylin–eosin (HE) staining of (B) UMUC-3 tumour treated by vehicle control and (C) that of tumour treated with 1 mg kg−1 of BMA. TUNEL staining of (D) UMUC-3 tumour treated by vehicle control and (E) tumour treated with BMA (bars=100 μm).
Discussion
Prognostic significance of Bcl-xL in UTUC human samples
Apoptosis-related molecular markers are important for predicting the biological behavior of cancer cells, including UC cells. Deregulation of apoptosis-related proteins has been reported to have a central role in oncogenesis and cancer progression in bladder cancer (Shariat et al, 2004; Karam et al, 2007). The assessment of combined apoptosis markers could help identify those patients at high risk for disease recurrence and mortality.
The associations between apoptosis-related proteins, including p53, Bcl-2, Bax, and survivin, in UTUC have been previously reported. The molecular marker p53 is the most investigated marker in bladder carcinoma; however, there is insufficient evidence to conclude that p53 acts as a marker of outcome (Malats et al, 2005). In UTUC patients, several studies reported that overexpression of p53 is related to increased tumour proliferation and disease progression and survival (Rey et al, 1997; Zigeuner et al, 2004; Nakanishi et al, 2005). On the other hand, other studies reported that there was no relationship between p53 expression and the prognosis in UTUC patients (Fromont et al, 2005; Jeong et al, 2009). Therefore, whether the expression of p53 or the function has prognostic value in UTUC patients is still controversial. Bcl-2 was also investigated in several studies to determine whether it is a significant prognostic factor in UTUC patients. However, the results of these studies suggested that there was no significant association between Bcl-2 expression and the prognosis of UTUC patients (Nakanishi et al, 1998; Korkolopoulou et al, 2002; Jeong et al, 2009). Jeong et al (2009) evaluated the expression of Bax in UTUC specimens and reported that Bax expression was not associated with the clinical outcome of patients with UTUC. Survivin expression in UTUC tissues was also evaluated in several studies. However, the evaluation of survivin and its prognostic significance are controversial (Nakanishi et al, 2002; Jeong et al, 2009). Therefore, the prognostic significance of these apoptosis-related markers has not yet been fully characterised in patients with UTUC.
Bcl-xL is a pro-survival member of the Bcl-2 family and has an important role in regulating cell survival and apoptosis. Bcl-xL has been found to be overexpressed in many human cancers, and its prognostic role was investigated in several studies, the results of which suggest Bcl-xL overexpression may indicate a poor prognosis for patients (Watanabe et al, 2004; Williams et al, 2005; Karczmarek-Borowska et al, 2006; Soltani-Arabshahi et al, 2009). Recently, Jin-Song et al (2012) reported that the level of Bcl-xL protein expression was closely correlated with tumour characteristics in colorectal cancer patients, and patients with high Bcl-xL expression showed poorer overall survival than those with low Bcl-xL expression. Differences in the intensity of Bcl-xL expression in tumour specimens may have an effect on the control of cancer cell death. Therefore, these data suggest that Bcl-xL expression or its intensity have an important role as a significant molecular marker in human malignancies. Bcl-xL expresses in high frequency in UC of urinary bladder; however, it is unclear whether there is a relationship between the prognosis of bladder cancer and the expression of Bcl-xL (Korkolopoulou et al, 2002). Taking into consideration such a result, we decided to evaluate the prognosis of UTUC patients using the expression level of Bcl-xL protein in tissue obtained from the patients.
In the present study, we investigated 175 cases of UTUC and analysed the significance of the expression of pro-survival Bcl-xL protein by immunohistochemistry. Our results indicated that a high Bcl-xL score in tumour specimens was an independent prognostic indicator for CSS in UTUC patients. When we analysed the clinicopathological data in the subgroup of patients with a higher pathological stage, higher tumour grade, or LVI positive, a high Bcl-xL score was significantly associated with a worse CSS. These results indicated that the deregulation of pro-survival pathways in UTUC is significantly associated with cancer progression and Bcl-xL may become a useful prognostic marker, which might lead to the identification of patients who stand to benefit from intensified therapy such as adjuvant chemotherapy and might provide an appropriate monitoring schedule with appropriate follow-up. To the best of our knowledge, this is the first report to examine the prognostic significance of Bcl-xL expression in a large number of UTUC cases.
Therapeutic efficacy of targeting Bcl-xL using BMA in vitro and in vivo
Our clinicopathological results suggest that targeting Bcl-xL protein seems to be a reasonable approach for assembling therapeutic strategies for the treatment of UC patients to improve their prognosis. Using siRNA targeting Bcl-xL, we demonstrated anti-cancer effect in bladder cancer cells. Therefore Bcl-xL is to be one of the important molecules for bladder cancer cell survival. Kunze et al (2012) reported that simultaneous siRNA-mediated knockdown of antiapoptotic protein mediated a significant reduction in cell viability and induction of apoptosis, suggesting Bcl-xL itself can be targeted for treatment. Therefore, clinically achievable molecules targeting Bcl-xL are needed for cancer therapy. In the present study, we focused on V-ATPase inhibitors, which can disturb the anti-apoptotic function of Bcl-xL. BMA has demonstrated an anti-cancer effect in several malignancies (Ohta et al, 1998; Nakashima et al, 2003; Morimura et al, 2008) and been shown to regulate the function of Bcl-xL protein in cancer cells in lung cancer cell lines (Sasazawa et al, 2009). Sasazawa et al (2009) suggested that BMA, a potent functional suppressor of Bcl-xL, functions not as a small molecule that can inactivate by recognising the surface pocket of Bcl-xL but rather as a modulator of cellular pH which induces the alternation between pro- and anti-apoptotic protein function. The role of V-ATPase in cell death and the mechanisms of apoptosis in cancer cells are still not fully elucidated. Recently, von Schwarzenberg et al (2013) reported that V-ATPase inhibitors induce the energy starvation stress response with a decline in the ATP level in the cellular environment before induction of cell apoptosis. Dysfunction of V-ATPase induced by an inhibitor may affect intracellular homeostasis supported by an appropriate pH balance. Our experiments demonstrated that the Bcl-xL was an important molecule for cancer cell survival; however, mainly reported the effect of BMA treatment and did not investigatd specifically whether BMA really targets only Bcl-xL protein. BMA, which is a potent inhibitor of V-ATPase, has several functions for cancer cells and its cell survival control. Therefore, the role as the Bcl-xL inhibitor may be one of the most important functions of BMA in cancer treatment. Further investigations are needed to clarify the interactions between BMA and Bcl-xL protein.
No study has evaluated whether targeting therapy for Bcl-xL proteins by BMA could induce apoptosis or whether it has an anti-tumour effect in UC cells. Our in vitro study demonstrated that BMA inhibited UMUC-3 cell proliferation by induction of apoptosis through a caspase-dependent pathway. In vivo study revealed that BMA significantly inhibited tumour growth compared with the control group. Ohta et al (1998) reported that the administration of BMA significantly inhibited tumour growth compared with control in nude mice bearing a xenografted pancreatic tumour cell line. Our data also demonstrate the effectiveness of xenograft tumour treatment with UC cells without any toxicity. Furthermore, TUNEL staining demonstrated that BMA induced apoptosis in UMUC-3 xenograft tumours in vivo, a finding that was compatible with the in vitro study results. We also analysed histologically the major organs, including liver, kidney, and spleen, by preparing HE-stained sections and found that there were no significant differences in the histological appearance of or damage to these organs between the control and treated mice. However, it is still unknown how BMA regulates the expression of Bcl-xL protein in cancer cells, and continuous effort is needed to delineate the precise mechanisms of apoptosis caused by BMA in vitro and in vivo.
In conclusion, the findings of the present study indicate that Bcl-xL expression can predict the survival of UTUC patients. Therapy that targets Bcl-xL may be a promising therapeutic strategy for patients with UC.
Change history
11 June 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB (1993) bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74: 597–608
Bolenz C, Weiss C, Wenzel M, Gabriel U, Steidler A, Becker A, Herrmann E, Trojan L, Michel MS (2009) In vivo evaluation of intravesical paclitaxel and combined bcl-xL antisense oligodeoxynucleotide treatment for orthotopic urothelial carcinoma. J Cancer Res Clin Oncol 135: 679–686
Catto JW, Yates DR, Rehman I, Azzouzi AR, Patterson J, Sibony M, Cussenot O, Hamdy FC (2007) Behavior of urothelial carcinoma with respect to anatomical location. J Urol 177: 1715–1720
Cotter TG (2009) Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer 9: 501–507
Epstein JI, Amin MB, Reuter VR, Mostofi FK (1998) The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol 22: 1435–1448
Fromont G, Roupret M, Amira N, Sibony M, Vallancien G, Validire P, Cussenot O (2005) Tissue microarray analysis of the prognostic value of E-cadherin, Ki67, p53, p27, survivin and MSH2 expression in upper urinary tract transitional cell carcinoma. Eur Urol 48: 764–770
Gabriel U, Bolenz C, Becker A, Schaaf A, Steidler A, Trojan L, Weiss C, Michel MS (2008) Evaluation of cytotoxic effects induced by bcl-2 and bcl-xL antisense-oligodeoxynucleotides in normal urothelium and transitional cell carcinoma. Oncol Rep 20: 1419–1423
Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG (1998) Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology 52: 594–601
Jeong IG, Kim SH, Jeon HG, Kim BH, Moon KC, Lee SE, Lee E (2009) Prognostic value of apoptosis-related markers in urothelial cancer of the upper urinary tract. Hum Pathol 40: 668–677
Jin-Song Y, Zhao-Xia W, Cheng-Yu L, Xiao-Di L, Ming S, Yuan-Yuan G, Wei D (2012) Prognostic significance of Bcl-xL gene expression in human colorectal cancer. Acta Histochem 113: 810–814
Kang MH, Reynolds CP (2009) Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res 15: 1126–1132
Karam JA, Lotan Y, Karakiewicz PI, Ashfaq R, Sagalowsky AI, Roehrborn CG, Shariat SF (2007) Use of combined apoptosis biomarkers for prediction of bladder cancer recurrence and mortality after radical cystectomy. Lancet Oncol 8: 128–136
Karczmarek-Borowska B, Filip A, Wojcierowski J, Smolen A, Korobowicz E, Korszen-Pilecka I, Zdunek M (2006) Estimation of prognostic value of Bcl-xL gene expression in non-small cell lung cancer. Lung Cancer 51: 61–69
Kashibuchi K, Tomita K, Schalken JA, Kume H, Yamaguchi T, Muto S, Horie S, Kitamura T (2006) The prognostic value of E-cadherin, alpha-, beta-, and gamma-catenin in urothelial cancer of the upper urinary tract. Eur Urol 49: 839–845
Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, Cheng L, Kiemeney L, Kriegmair M, Montironi R, Murphy WM, Sesterhenn IA, Tachibana M, Weider J (2005) Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 66: 4–34
Kirsh EJ, Baunoch DA, Stadler WM (1998) Expression of bcl-2 and bcl-X in bladder cancer. J Urol 159: 1348–1353
Korkolopoulou P, Lazaris A, Konstantinidou AE, Kavantzas N, Patsouris E, Christodoulou P, Thomas-Tsagli E, Davaris P (2002) Differential expression of bcl-2 family proteins in bladder carcinomas. Relationship with apoptotic rate and survival. Eur Urol 41: 274–283
Kosaka T, Kikuchi E, Mikami S, Miyajima A, Shirotake S, Ishida M, Okada Y, Oya M (2010) Expression of snail in upper urinary tract urothelial carcinoma: prognostic significance and implications for tumor invasion. Clin Cancer Res 16: 5814–5823
Krajewska M, Krajewski S, Epstein JI, Shabaik A, Sauvageot J, Song K, Kitada S, Reed JC (1996) Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am J Pathol 148: 1567–1576
Kunze D, Kraemer K, Erdmann K, Froehner M, Wirth MP, Fuessel S (2012) Simultaneous siRNA-mediated knockdown of antiapoptotic BCL2, Bcl-xL, XIAP and survivin in bladder cancer cells. Int J Oncol e-pub ahead of print 6 July 2012; doi:10.3892/ijo.2012.1549
Lebedeva I, Raffo A, Rando R, Ojwang J, Cossum P, Stein CA (2001) Chemosensitization of bladder carcinoma cells by bcl-xL antisense oligonucleotides. J Urol 166: 461–469
Malats N, Bustos A, Nascimento CM, Fernandez F, Rivas M, Puente D, Kogevinas M, Real FX (2005) P53 as a prognostic marker for bladder cancer: a meta-analysis and review. Lancet Oncol 6: 678–686
Miyata Y, Kanda S, Nomata K, Eguchi J, Kanetake H (2005) Expression of cyclooxygenase-2 and EP4 receptor in transitional cell carcinoma of the upper urinary tract. J Urol 173: 56–60
Morimura T, Fujita K, Akita M, Nagashima M, Satomi A (2008) The proton pump inhibitor inhibits cell growth and induces apoptosis in human hepatoblastoma. Pediatr Surg Int 24: 1087–1094
Nakanishi K, Hiroi S, Tominaga S, Aida S, Kasamatsu H, Matsuyama S, Matsuyama T, Kawai T (2005) Expression of hypoxia-inducible factor-1alpha protein predicts survival in patients with transitional cell carcinoma of the upper urinary tract. Clin Cancer Res 11: 2583–2590
Nakanishi K, Tominaga S, Hiroi S, Kawai T, Aida S, Kasamatsu H, Aurues T, Hayashi T, Ikeda T (2002) Expression of survivin does not predict survival in patients with transitional cell carcinoma of the upper urinary tract. Virchows Arch 441: 559–563
Nakanishi K, Tominaga S, Kawai T, Torikata C, Aurues T, Ikeda T (1998) Expression of bcl-2 oncoprotein in transitional cell carcinoma of the upper urinary tract. Virchows Arch 432: 445–450
Nakashima S, Hiraku Y, Tada-Oikawa S, Hishita T, Gabazza EC, Tamaki S, Imoto I, Adachi Y, Kawanishi S (2003) Vacuolar H+-ATPase inhibitor induces apoptosis via lysosomal dysfunction in the human gastric cancer cell line MKN-1. J Biochem 134: 359–364
Ohta T, Arakawa H, Futagami F, Fushida S, Kitagawa H, Kayahara M, Nagakawa T, Miwa K, Kurashima K, Numata M, Kitamura Y, Terada T, Ohkuma S (1998) Bafilomycin A1 induces apoptosis in the human pancreatic cancer cell line Capan-1. J Pathol 185: 324–330
Oosterlinck W (2007) Ureteral tumour: a specific upper urinary tract transitional cell carcinoma. Eur Urol 51: 1164–1165
Rey A, Lara PC, Redondo E, Valdes E, Apolinario R (1997) Overexpression of p53 in transitional cell carcinoma of the renal pelvis and ureter. Relation to tumor proliferation and survival. Cancer 79: 2178–2185
Rink M, Ehdaie B, Cha EK, Green DA, Karakiewicz PI, Babjuk M, Margulis V, Raman JD, Svatek RS, Fajkovic H, Lee RK, Novara G, Hansen J, Daneshmand S, Lotan Y, Kassouf W, Fritsche HM, Pycha A, Fisch M, Scherr DS, Shariat SF (2012) Stage-specific impact of tumor location on oncologic outcomes in patients with upper and lower tract urothelial crcinoma following radical surgery. Eur Urol 62: 677–684
Sasazawa Y, Futamura Y, Tashiro E, Imoto M (2009) Vacuolar H+-ATPase inhibitors overcome Bcl-xL-mediated chemoresistance through restoration of a caspase-independent apoptotic pathway. Cancer Sci 100: 1460–1467
Shariat SF, Tokunaga H, Zhou J, Kim J, Ayala GE, Benedict WF, Lerner SP (2004) p53, p21, pRB, and p16 expression predict clinical outcome in cystectomy with bladder cancer. J Clin Oncol 22: 1014–1024
Soltani-Arabshahi R, Leboeuf C, Rivet J, Pisonero H, Zhao WL, Bachelez H, Ameisen JC, Janin A (2009) Bcl-xL gene expression correlated with lower apoptotic cell numbers and shorter progression-free survival in PCFCL. J Invest Dermatol 129: 1703–1709
von Schwarzenberg K, Wiedmann RM, Oak P, Schulz S, Zischka H, Wanner G, Efferth T, Trauner D, Vollmar AM (2013) Mode of cell death induction by pharmacological vacuolar H+-ATPase (V-ATPase) inhibition. J Biol Chem 288: 1385–1396
Watanabe J, Kushihata F, Honda K, Sugita A, Tateishi N, Mominoki K, Matsuda S, Kobayashi N (2004) Prognostic significance of Bcl-xL in human hepatocellular carcinoma. Surgery 135: 604–612
Williams J, Lucas PC, Griffith KA, Choi M, Fogoros S, Hu YY, Liu JR (2005) Expression of Bcl-xL in ovarian carcinoma is associated with chemoresistance and recurrent disease. Gynecol Oncol 96: 287–295
Zigeuner R, Tsybrovskyy O, Ratschek M, Rehak P, Lipsky K, Langner C (2004) Prognostic impact of p63 and p53 expression in upper urinary tract transitional cell carcinoma. Urology 63: 1079–1083
Acknowledgements
This work was supported, in part, by Grants-in-Aid for Scientific Research (to EK), Grant-in-Aid for Young Scientists (to TK), and Grant-in-Aid for Young Scientists (to SY) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Yoshimine, S., Kikuchi, E., Kosaka, T. et al. Prognostic significance of Bcl-xL expression and efficacy of Bcl-xL targeting therapy in urothelial carcinoma. Br J Cancer 108, 2312–2320 (2013). https://doi.org/10.1038/bjc.2013.216
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.216
Keywords
This article is cited by
-
Prognostic factors and predictive tools for upper tract urothelial carcinoma: a systematic review
World Journal of Urology (2017)
-
Targeted therapies in bladder cancer: an overview of in vivo research
Nature Reviews Urology (2015)