Abstract
Background:
CD151 is a member of the tetraspanin family, which interacts with laminin-binding integrins and other tetraspanins. This protein is implicated in motility, invasion, and metastasis of cancer cells, but the prevalence of CD151 expression in subtypes of breast cancers and its influence on clinical outcome remains to be evaluated.
Methods and results:
The immunohistochemistry-based tissue microarray analysis showed that 127 (14.3%) cases overexpressed CD151 among 886 breast cancer patients. CD151 overexpression was found to be significantly associated with larger tumour size, higher nodal stage, advanced stage, absence of oestrogen receptor and progesterone receptor, and human epidermal growth factor receptor 2 overexpression. CD151 overexpression resulted in poorer overall survival (OS) (P<0.001) and disease-free survival (P=0.02), and stage II and III patients with CD151 overexpression demonstrated substantially poorer OS (P=0.0474 and 0.0169). In the five subtypes analyses, CD151 overexpression retained its adverse impact on OS in the Luminal A (P=0.0105) and quintuple-negative breast cancer (QNBC) subtypes, one subgroup of triple-negative breast cancer (P=0.0170). Multivariate analysis that included stage, subtype, and adjuvant chemotherapy showed that CD151 overexpression was independently associated with poor OS in invasive breast cancer.
Conclusion:
CD151 overexpression may be a potential molecular therapeutic target for breast cancer, especially in QNBC subtype and more advanced stages of breast cancer.
Similar content being viewed by others
Main
CD151 is a member of the mammalian tetraspanins, which are transmembrane proteins involved in a variety of biological processes including the immune system, fertilisation, infectious processes, and tumour progression (Maecker et al, 1997; Hemler, 2008). Tetraspanin proteins form complexes between themselves or with other non-tetraspanin molecules such as integrins, immunoglobulin superfamily members, and signalling molecules, and carry out several functions depending on interacting partners (Hemler, 2005; Zoller, 2009). Particularly, CD151 contributes to integrin-dependent cell adhesion and motility by directly interacting with laminin-binding integrins (α3β1, α6β1, α6β4, and α7β1) (Hemler, 2008). A recent study also reported that CD151 has a role in proliferation of mammalian epithelial cells, suggesting that CD151 may contribute to the tumour cell growth (Novitskaya et al, 2010). Tetraspanin CD151 is expressed in most of cells and tissue types showing high expression in epithelial and endothelial cells (Sincock et al, 1997; Zoller, 2009). Deregulation of several tetraspanins is observed in human cancer, and these upregulation or downregulation are of clinical significance in some malignancies (Romanska and Berditchevski, 2011). Upregulation of CD151 is found in many tumour types and CD151 overexpression was associated with poor prognosis in non-small cell lung (Tokuhara et al, 2001), colon cancer (Hashida et al, 2003), hepatocellular (Ke et al, 2009), pancreatic (Zhu et al, 2011), oesophageal (Suzuki et al, 2010), and endometrial cancer (Voss et al, 2011). In addition, there have been several evidences supporting the contribution of CD151 in tumour progression. Although tetraspanins CD82 and CD9 are known to suppress metastasis (Zoller, 2009), CD151 promotes metastasis by regulating tumour cell migration (Zijlstra et al, 2008). Specifically, overexpression of CD151 enhances cell motility, invasion, and metastasis in colon cancer and fibrosarcoma cells (Kohno et al, 2002). In hepatocelluar carcinoma cells, CD151 expression promotes invasiveness of tumour cells in association with induction of epithelial–mesenchymal transitions (Ke et al, 2011).
Based on the association of α6β4 integrin in mammary tumourigenesis (Shaw et al, 1997; Zahir et al, 2003; Guo et al, 2006), the relevance of CD151 in breast cancer was also hypothesised. Indeed, Yang et al (2008) showed that CD151 expression is elevated in breast cancer, with even more upregulation in high-grade and oestrogen-negative subtypes including basal-like breast cancer. Moreover, it was demonstrated that loss of CD151 decreased the integrin-mediated cell migration, spreading, invasion, and signalling (through FAK, Rac1, and lck) of basal-like mammary cell lines with the effect on the subcellular distribution of α6 integrins (Yang et al, 2008). The delayed breast cancer progression by CD151 ablation was also shown in mouse xenograft models established using basal-like cell line, suggesting that CD151 may be a novel therapeutic target in certain breast cancer subtypes (Yang et al, 2008). High expression of CD151 in high-grade breast cancer was also confirmed in the recent study by Sadej et al (2009). Furthermore, in the same study, CD151 overexpression was shown to correlate with decreased survival of patients with breast cancer when assessed in 56 cases (Sadej et al, 2009). However, the association of CD151 expression with clinical outcome as well as its significance as prognostic factor in breast cancer patients is still unclear. Moreover, a systematic approach examining the incidence of CD151 expression and the significance of CD151 on clinical outcomes in breast cancer subtypes has not been undertaken. In order to select the appropriate breast cancer patients for targeted therapy, a detailed analysis using marker-driven subtyping in patient populations is critical. Therefore, to define the prognostic impact of CD151 expression in breast cancer subtypes, we divided 886 patients with breast cancer into five subtypes and assessed the relationship of CD151 expression with clinical outcome including overall survival (OS) and disease-free progression survival in each subtype.
Materials and Methods
Study samples and five subtypes information
A tissue microarray (TMA) constructed from duplicate 2 mm cores of invasive breast carcinomas from 1290 primary invasive breast cancer samples was utilised for the analysis of CD151 status. This retrospective cohort was named ‘Samsung Medical Center Breast Cancer Biomarker Study' and was originally intended for the clinical validation of a novel biomarker set according to the breast cancer subtype (Choi et al, 2010). The clinical features of this cohort were as follows: (1) patients did not receive cytotoxic chemotherapy or hormones before surgery; (2) all oestrogen receptor (ER)-positive patients underwent hormonal therapy with tamoxifen; (3) none of the patients underwent anti-human epidermal growth factor receptor 2 (HER2) therapy. The pathological tumour stage was assessed according to the American Joint Committee on Cancer (AJCC) 6 Staging System. The histological grade was determined according to the Bloom–Richardson classification scheme. This study was approved by the Institutional Review Board at the Samsung Medical Center (Seoul, Korea) in accordance with the Declaration of Helsinki.
Only 951 of the cases had subtype information (Choi et al, 2010). Each case was divided into five subgroups according to the status of ER, progesterone receptor (PR), HER2, and basal markers (either epidermal growth factor receptor (EGFR) or cytokeratin 5/6 (CK5/6)) as described previously (Choi et al, 2010): (1) Luminal A (ER+ or PR+/HER2−), (2) Luminal B (ER+ or PR+/HER2+), (3) HER2 (ER−/PR−/HER2+), triple-negative breast cancer (TNBC, ER−/PR−/HER2−), TNBC subtype was further divided into (4) basal-like breast cancer (BLBC, ER−/PR−/HER2−/EGFR+ or CK5/6+), and (5) quintuple-negative breast cancer (QNBC, ER−/PR−/HER2−/EGFR−/CK5/6−) (Choi et al, 2010). Although the ER and PR status were acquired from the pathological report using the semi-quantitative Allred score, the HER2, CK5/6, and EGFR status were determined from the TMA analysis. Tumours were classified as HER2 positive if they had a score of 3+ in regard to the staining on IHC and/or gene amplification as determined by fluorescence in situ hybridisation when using HER2, such that the chromosome 17 ratio was >2.2. Cytokeratin 5/6 was interpreted as positive if there was any observation of cytoplasm and/or membranous staining. The EGFR status was scored as positive when at least 10% of the tumour cells showed strong membranous staining.
Immunohistochemical analysis
Immunohistochemical analyses were performed on the paraffin sections as described previously (Chien et al, 2008). The TMA sections were incubated with the monoclonal mouse anti-human CD151 antibody at room temperature for 60 min (1 : 100 dilution, RLM30, Novocastra, Newcastle upon Tyne, UK). Specimens were then incubated with a 1 : 1000 dilution of biotinylated goat anti-mouse IgG (Vector Laboratories, Burlingame, CA, USA) for 1 h at room temperature after washing with PBS. CD151 expression was scored using the HER2 semi-quantitative method based on the following four classes (Wolff et al, 2007): score 0 (no staining is observed or cell membrane staining is observed in <10% of the tumour cells), score 1+ (a faint perceptible membrane staining can be detected in >10% of the tumour cells. The cells are only stained in parts of their membrane), score 2+ (a weak to moderate complete membrane staining is observed in >10% of the tumour cells), and score 3+ (a strong complete membrane staining is observed in >30% of the tumour cells). Scores ranging from 0 to 2+ were classified as CD151-low expression and cases that had a score of 3+ were classified into the CD151-high expression group. Two pathologists (MJK and YLC) independently scored the immunohistochemical staining and were blinded with respect to the results of the other markers and the outcome data.
Statistical analysis
Disease-free survival (DFS) was defined as the time from the date of diagnosis to the date of documented relapse, including locoregional recurrence and distant metastasis. Overall survival was expressed as the number of months from diagnosis of breast cancer to the date of death. Differences in the frequencies of the basic characteristics, clinical parameters, and subtypes were statistically analysed using either the chi-square test or the Fisher's exact test in cases when the expected values of any of the cells were <5. Survival curves were constructed using the Kaplan–Meier method and the log-rank test was used to compare the mean survival rates across the groups. The log-rank test with Bonferroni's correction was used for the subgroup survival analysis. For the multivariate analysis, Cox regression models were constructed in order to estimate the adjusted hazard ratios (HRs) of the groups according to stage, adjuvant chemotherapy, and subtype. P-values <0.05 were considered to be statistically significant and all of the P-values corresponded to two-sided significance tests. All of the statistical analyses were performed using SPSS 16.0 (Chicago, IL, USA). The ‘REMARK’ criteria of the National Cancer Institute was used in the design, analysis, and interpretation of the results (McShane et al, 2005).
Results
Patient characteristics and CD151 expression
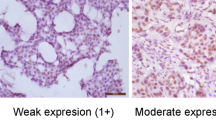
In 65 cases, CD151 stain was considered to be unsatisfactory because of loss of tissue core or no invasive cancer component, and these cases were further excluded from 951 cases with subtype information. Therefore, a total of 886 cases with informative immunohistochemical results were included in this analysis. All of the patients were Korean females who had curative resection of their primary tumours and axillary node dissection or sentinel node sampling. The median age at diagnosis was 46 years (range, 23–80 years). The characteristics of the patients are provided in Table 1. In the normal breast tissue, CD151 was expressed in the basal-myoepithelial cell layer surrounding both ducts and tubule-lobular units (Figures 1A and B). The invasive cancers showed CD151 expression predominantly localised to the membrane, with expression occurring in the cytoplasm in some cases (Figures 1D, E and F). The numbers of patients in each group of CD151 expression were as follows: score 0, 80 (9.0%); score 1, 356 (40.2%); score 2, 323 (36.5%); and score 3, 127 (14.3%). In all, 127 (14.3%) cases were identified as CD151-high expression and 759 (85.7%) cases were classified as CD151-low expression. CD151 overexpression was significantly associated with a more advanced stage (P<0.001), larger tumour size (P<0.001), lymph node involvement (P<0.001), and absence of ER (P<0.001) and PR (P=0.009) (Table 1). There were no significant differences in the distribution of adjuvant chemotherapy modalities between the CD151-low group and the CD151-high group (P=0.409). CD151 overexpression was detected more frequently in breast cancers with HER2 overexpression (21.9%) than in HER2-negative breast cancers (11.8%, P<0.001). When CD151 overexpression was compared among the breast cancer subtypes (Luminal A, Luminal B, HER2, BLBC, and QNBC), CD151 overexpression varied significantly according to the breast cancer subtype (P<0.001). The Luminal A subtype had a lower incidence in tumours with CD151 expression. CD151 overexpression was most frequent in the HER2 subtype (27.4%) (Table 1).
CD151 expression in normal tubule-lobular unit (A) and duct (B) in breast tissue ( × 200). CD151 expression is localised to the cytoplasm of the basal layer. Representative cases of each score of CD151 in invasive breast cancer (C, score 0; D, score 1; E, score 2; F, score 3, × 200). The strong membranous overexpression of CD151 is noted in invasive breast cancer (F).
Impact of CD151 overexpression on survival in breast cancer according to stage and subtype
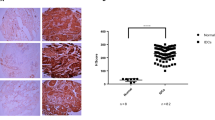
The time of DFS ranged from 0 to 148.7 months with a median of 68.8 months. During the study period, 24.6% of the women (218 out of 886) had local recurrence and/or metastasis. Duration of OS ranged from 6.0 to 148.7 months with a median of 74.9 months. During the study period, 14.1% of women (125 out of 886) died, whereas the remaining 761 were still alive at the end of the study. The breast cancer patients with CD151 overexpression demonstrated substantially poorer OS (CD151-high vs CD151-low; 109.8 months (95% confidence interval (CI), 100.9–118.7 months) vs 134.1 months (95% CI, 131.3–137.0 months), P<0.001) and DFS (CD151-high vs CD151-low; 104.2 months (95% CI, 94.6–113.7 months) vs 120.0 months (95% CI, 116.2–123.7 months), P=0.020) (Figure 2A). We performed survival analyses according to the AJCC stage of the breast cancer. Although CD151 overexpression did not show any impact on survival in regard to AJCC stage I cancer, CD151 overexpression had a significant influence on OS in stage II cancer (CD151-high vs CD151-low, 117.3 months (95% CI, 106.2–128.4 months) vs 135.6 months (95% CI, 131.7–139.5 months), P=0.0474) and stage III cancer (CD151-high vs CD1515-low, 86.7 months (95% CI, 70.9–102.5 months) vs 110.4 months (103.1–117.7 months), P=0.0169) (Figure 2B). We also performed subgroup analyses according to breast cancer subtype. CD151 overexpression did not markedly influence OS in the Luminal B or HER2 subtypes (Figure 3). Luminal A subtype with CD151 overexpression showed a significantly poor OS (CD151-high vs CD151-low, 109.4 months (95% CI, 97.5–121.4) vs 139.6 months (95% CI, 136.2–143.0); P=0.0105) (Figure 3). The TNBC subtype with CD151 overexpression had a more rapid deteriorating clinical course (median OS, 91.6 months (95% CI, 76.8–106.5)) compared with that of CD151-low patients with TNBCs (median OS, 126.9 months (95% CI, 120.9–133.0)), in terms of OS (P=0.010) (Figure 3).
Next, the prognostic value of CD151 expression was evaluated in the subgroup analyses according to five subtypes as the QNBC subtype was expected to be insensitive to chemotherapy. CD151 overexpression did not significantly affect OS in BLBCs with CD151 overexpression, but a trend toward poorer OS did exist (CD151-high vs CD151-low, 99.7 months (95% CI, 81.5–117.8) vs 127.7 months (95% CI, 120.2–135.2); P=0.615). In regard to the QNBC subtype, CD151 overexpression retained its significant adverse impact on OS (CD151-high vs CD151-low, 74.9 months (95% CI, 54.0–95.7) vs 123.7 months (95% CI, 113.6–133.8); P=0.0170) (Figure 3).
Prognostic factor analyses
The factors that predicted poor OS based on the univariate analysis were CD151 overexpression, AJCC stage, cancer subtype, and adjuvant chemotherapy (Table 2). CD151 overexpression, AJCC stage, and subtype were significant prognostic factors for DFS. In the Cox regression model, the prognostic factors for OS in all of the patients were AJCC stage, breast cancer subtype, adjuvant chemotherapy, and CD151 overexpression. In regard to the DFS, only the AJCC stage retained its statistical significance at the multivariate level (Table 3). Breast cancer patients with CD151 overexpression demonstrated a substantially lower OS with a 1.65-fold (P=0.034; HR, 1.65; 95% CI, 1.03–2.59) higher risk of death after adjusting for AJCC stage, breast subtype, and adjuvant chemotherapy (Table 3).
Discussion
The detailed clinical significance of CD151 overexpression in a large cohort of patients with breast cancer has not been previously reported. In this study of 886 breast cancer cases, we found that CD151 overexpression is an independent negative predictor of OS in patients with breast cancer and its worse impact on OS was retained in Luminal A and QBNC subtypes.
The normal expression of CD151 in the breast tissue was limited to the basal-myoepithelial cell layer surrounding both ducts and lobular alveolae, which agrees with the findings of a previous report (Yang et al, 2008). By contrast, variable patterns of CD151 expression were seen in the invasive breast cancer tissues, which ranged from absence to diffuse, strong overexpression occurred mainly in the membrane and/or cytoplasm of the tumour cells. Yang et al (2008) have detected the significant associations between CD151 expression and tumour grade, ER status, and combination of ER/HER2 status in 124 breast cancer cases. This is consistent with our findings because the ER-negative breast cancers, which had a higher proportion of CD151 overexpression, contained both HER2 and TNBC subtypes. Our data also indicated that the HER2 subtype had elevated CD151 expression, and that the Luminal A subtype had the lowest proportion of CD151 expression. However, they did not examine the long-term outcome or recurrence in their study, and they had calculated the CD151 positivity as 31% (Yang et al, 2008), whereas we calculated it as 14.3% in our study. These differences in the cutoff values to identify positive cases may be controversial. However, the clinicopathological characteristics of CD151 overexpression cases were similar in both cohorts.
On the other hand, it was found in a previous study by Sadej et al (2009) that CD151 overexpression in breast cancers is associated with decreased OS based on 56 cases of breast invasive ductal carcinomas, 30.4% of which were classified as being CD151 positive. Furthermore, they have shown that CD151 expression is also positively associated with the involvement of regional lymph nodes. However, there were no associations between CD151 expression and ER status, tumour grade, disease stage, and age. Compared with these two previous studies of CD151 in breast cancer, our study utilised a larger number of cases that had sufficient follow-up data, including subtype analysis and thus this might be a reason for the discrepancy in results between this study and previous studies.
The upregulation of CD151 expression has been seen in many types of tumours and is generally associated with a poor prognosis (Romanska and Berditchevski, 2011). The positive rate or proportion of CD151 overexpression that is detectable by immunohistochemistry in other cancers is variable (Table 4). Furthermore, there is no consensus for the cutoff criteria of CD151 expression. We used the scoring method of HER2 in a semi-quantitative manner and cases with a score of 3+ were considered to have CD151-high expression. CD151 overexpression occurred mainly in the membrane and/or cytoplasm in the tumour cells of the cases. As CD151 is a transmembrane protein, its functional localisation is believed to be the cellular membrane. Therefore, we did not include cases that showed only cytoplasmic expression, which was rare in our CD151-high expression group.
In agreement with poor prognosis of CD151 overexpression in several cancer types, CD151 is a metastasis-promoting tetraspanin protein. Actually, CD151-transfected cancer cell lines enhanced cell migration and invasion (Kohno et al, 2002; Ang et al, 2010; Ke et al, 2011). The involvement of CD151 through regulation of cell motility in metastasis was also demonstrated in vivo (Zijlstra et al, 2008). Additionally, anti-CD151 antibody treatment of high-CD151-expressing tumour cells decreased cell migration and metastasis (Testa et al, 1999). CD151-blocking antibody was reported to inhibit invasion and intravasation at the site of the primary tumour (Zijlstra et al, 2008). Moreover, a recent in vivo study showed that CD151-null mice have markedly diminished experimental lung metastasis (Sadej et al, 2010).
In our study, CD151 overexpression was also associated with poor prognosis of breast cancer patients in line with its clinical significance in other types of cancer. In particular, high-CD151 expression was significantly correlated with a larger tumour size, higher lymph node involvement, and advanced stage of invasive breast cancer. Its association with a larger tumour size in this study can be explained by the previous findings showing that CD151 has a positive role in breast tumour cell growth in vivo, whereas its downregulation causes an inhibition of tumour cell growth (Sadej et al, 2009). In addition, CD151 ablation was found to inhibit the migration, invasion, and spreading of breast cancer cells in relation with its effect on the subcellular distribution of integrins, suggesting the promoting role of CD151 in breast tumour progression (Yang et al, 2008). Furthermore, a recent study has shown that the depletion of CD151 attenuates pulmonary metastasis of breast cancer cells by regulating transforming growth factor β signalling (Sadej et al, 2010). These results may support the relevance of high-CD151 expression to a higher lymph node involvement and thereby advanced stage of invasive breast cancer in this study. Combination of these promoting effects of CD151 expression on breast cancer progression including tumour size and lymph node involvement may be responsible for a poor prognosis of breast cancer patients with high-CD151 expression.
However, CD151 overexpression was found to be an independent negative prognosis factor for OS but not for DFS of patients with breast cancer in this study. In regard to DFS, only the AJCC stage retained its statistical significance after adjustments for other prognostic factors including CD151 expression, subtypes, and adjuvant chemotherapy, suggesting that AJCC stage is a strong predictor of recurrence of breast cancer superior to other prognostic factors in this study. It is unclear why this difference in the effect of CD151 expression on the prediction of OS or DFS was observed. However, it is likely that CD151 expression influences the recurrence of breast cancer indirectly together with other variables, not in a direct manner, and thereby its effects on the prediction of DFS may not be as strong as AJCC stage even though high-CD151 expression was significantly correlated with recurrence of breast cancer patients in univariate analysis.
In this study, CD151 overexpression was found in 21.9% of the HER2-positive cases, which includes both HER2 and Luminal B subtypes, and was found in 27.4% of the HER2 subtype. Recently, it has been reported that CD151 is one of the mechanisms of resistance to anti-ErbB2 (HER2) agents, which suggests that targeting CD151 offers potential advantages such as drug sensitisation (Yang et al, 2010). Interestingly, although CD151 overexpression was observed most frequently in HER2 subtype, a significant effect on survival was not shown. However, we cannot make any definitive conclusions based on this result as our cohort did not receive anti-ErbB2 (HER2) therapy. The clinical significance of CD151 overexpression in the HER2 subtype should be investigated in a cohort that has received anti-ErbB2 (HER2) therapy. CD151-α6β4 integrin complexes may influence the sensitivity to ErbB2 (HER2)-targeted therapies as α6β4 enhances the signalling of ERBB family members (Guo et al, 2006). Constitutively activated proteins in these pathways may contribute to the clinical characteristics of HER2-positive tumours. Whether the CD151-associated HER2-positive breast cancer represents an independent disease entity remains unanswered and needs to be clarified in future studies. Thus, correlative analyses with CD151 and key proteins of these cascade pathways may be interesting to investigate so that the pathogenic role of CD151 in HER2-positive breast cancer is further clarified. CD151-high cases were rarest in the Luminal A subtype, but high-CD151 expression in this group was significantly associated with shorter OS. We also found that 16.2% of TNBC overexpressed CD151 and CD151-high cases showed a significantly poor OS consistent with the previous study demonstrating a role for CD151-α6 integrin complexes in basal-like breast cancer progression (Yang et al, 2008).
In conclusion, patients with CD151 expression have a more rapid deteriorating clinical course with poorer OS compared with those not expressing the protein. CD151 expression may be a potential molecular therapeutic target for breast cancer, especially in the Luminal A and QNBC subtypes, and advanced stages of cancers. Thus, more effective treatment should be adopted in this particular subset of patients by possibly administering CD151-targeted therapy during conventional chemotherapy and HER2-targeted therapy regimens.
Change history
29 March 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ang J, Fang BL, Ashman LK, Frauman AG ( 2010 ) The migration and invasion of human prostate cancer cell lines involves CD151 expression . Oncol Rep 24 (6) : 1593 – 1597
Ang J, Lijovic M, Ashman LK, Kan K, Frauman AG ( 2004 ) CD151 protein expression predicts the clinical outcome of low-grade primary prostate cancer better than histologic grading: a new prognostic indicator? Cancer Epidemiol Biomarkers Prev 13 (11 Pt 1) : 1717 – 1721
Chien CW, Lin SC, Lai YY, Lin BW, Lee JC, Tsai SJ ( 2008 ) Regulation of CD151 by hypoxia controls cell adhesion and metastasis in colorectal cancer . Clin Cancer Res 14 (24) : 8043 – 8051
Choi YL, Oh E, Park S, Kim Y, Park YH, Song K, Cho EY, Hong YC, Choi JS, Lee JE, Kim JH, Nam SJ, Im YH, Yang JH, Shin YK ( 2010 ) Triple-negative, basal-like, and quintuple-negative breast cancers: better prediction model for survival . BMC Cancer 10 : 507
Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, Giancotti FG ( 2006 ) Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis . Cell 126 (3) : 489 – 502
Hashida H, Takabayashi A, Tokuhara T, Hattori N, Taki T, Hasegawa H, Satoh S, Kobayashi N, Yamaoka Y, Miyake M ( 2003 ) Clinical significance of transmembrane 4 superfamily in colon cancer . Br J Cancer 89 (1) : 158 – 167
Hemler ME ( 2005 ) Tetraspanin functions and associated microdomains . Nat Rev Mol Cell Biol 6 (10) : 801 – 811
Hemler ME ( 2008 ) Targeting of tetraspanin proteins--potential benefits and strategies . Nat Rev Drug Discov 7 (9) : 747 – 758
Huang XY, Ke AW, Shi GM, Ding ZB, Devbhandari RP, Gu FM, Li QL, Dai Z, Zhou J, Fan J ( 2010 ) Overexpression of CD151 as an adverse marker for intrahepatic cholangiocarcinoma patients . Cancer 116 (23) : 5440 – 5451
Ke AW, Shi GM, Zhou J, Huang XY, Shi YH, Ding ZB, Wang XY, Devbhandari RP, Fan J ( 2011 ) CD151 amplifies signaling by integrin alpha6beta1 to PI3K and induces the epithelial-mesenchymal transition in HCC cells . Gastroenterology 140 (5) : 1629 – 1641e15
Ke AW, Shi GM, Zhou J, Wu FZ, Ding ZB, Hu MY, Xu Y, Song ZJ, Wang ZJ, Wu JC, Bai DS, Li JC, Liu KD, Fan J ( 2009 ) Role of overexpression of CD151 and/or c-Met in predicting prognosis of hepatocellular carcinoma . Hepatology 49 (2) : 491 – 503
Kohno M, Hasegawa H, Miyake M, Yamamoto T, Fujita S ( 2002 ) CD151 enhances cell motility and metastasis of cancer cells in the presence of focal adhesion kinase . Int J Cancer 97 (3) : 336 – 343
Maecker HT, Todd SC, Levy S ( 1997 ) The tetraspanin superfamily: molecular facilitators . FASEB J 11 (6) : 428 – 442
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM ( 2005 ) REporting recommendations for tumour MARKer prognostic studies (REMARK) . Br J Cancer 93 (4) : 387 – 391
Novitskaya V, Romanska H, Dawoud M, Jones JL, Berditchevski F ( 2010 ) Tetraspanin CD151 regulates growth of mammary epithelial cells in three-dimensional extracellular matrix: implication for mammary ductal carcinoma in situ . Cancer Res 70 (11) : 4698 – 4708
Romanska HM, Berditchevski F ( 2011 ) Tetraspanins in human epithelial malignancies . J Pathol 223 (1) : 4 – 14
Sadej R, Romanska H, Baldwin G, Gkirtzimanaki K, Novitskaya V, Filer AD, Krcova Z, Kusinska R, Ehrmann J, Buckley CD, Kordek R, Potemski P, Eliopoulos AG, Lalani el N, Berditchevski F ( 2009 ) CD151 regulates tumorigenesis by modulating the communication between tumor cells and endothelium . Mol Cancer Res 7 (6) : 787 – 798
Sadej R, Romanska H, Kavanagh D, Baldwin G, Takahashi T, Kalia N, Berditchevski F ( 2010 ) Tetraspanin CD151 regulates transforming growth factor beta signaling: implication in tumor metastasis . Cancer Res 70 (14) : 6059 – 6070
Shaw LM, Rabinovitz I, Wang HH, Toker A, Mercurio AM ( 1997 ) Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion . Cell 91 (7) : 949 – 960
Sincock PM, Mayrhofer G, Ashman LK ( 1997 ) Localization of the transmembrane 4 superfamily (TM4SF) member PETA-3 (CD151) in normal human tissues: comparison with CD9, CD63, and alpha5beta1 integrin . J Histochem Cytochem 45 (4) : 515 – 525
Suzuki S, Miyazaki T, Tanaka N, Sakai M, Sano A, Inose T, Sohda M, Nakajima M, Kato H, Kuwano H ( 2010 ) Prognostic significance of CD151 expression in esophageal squamous cell carcinoma with aggressive cell proliferation and invasiveness . Ann Surg Oncol 18 (3) : 888 – 893
Testa JE, Brooks PC, Lin JM, Quigley JP ( 1999 ) Eukaryotic expression cloning with an antimetastatic monoclonal antibody identifies a tetraspanin (PETA-3/CD151) as an effector of human tumor cell migration and metastasis . Cancer Res 59 (15) : 3812 – 3820
Tokuhara T, Hasegawa H, Hattori N, Ishida H, Taki T, Tachibana S, Sasaki S, Miyake M ( 2001 ) Clinical significance of CD151 gene expression in non-small cell lung cancer . Clin Cancer Res 7 (12) : 4109 – 4114
Voss MA, Gordon N, Maloney S, Ganesan R, Ludeman L, McCarthy K, Gornall R, Schaller G, Wei W, Berditchevski F, Sundar S ( 2011 ) Tetraspanin CD151 is a novel prognostic marker in poor outcome endometrial cancer . Br J Cancer 104 (10) : 1611 – 1618
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF ( 2007 ) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer . J Clin Oncol 25 (1) : 118 – 145
Yang XH, Flores LM, Li Q, Zhou P, Xu F, Krop IE, Hemler ME ( 2010 ) Disruption of laminin-integrin-CD151-focal adhesion kinase axis sensitizes breast cancer cells to ErbB2 antagonists . Cancer Res 70 (6) : 2256 – 2263
Yang XH, Richardson AL, Torres-Arzayus MI, Zhou P, Sharma C, Kazarov AR, Andzelm MM, Strominger JL, Brown M, Hemler ME ( 2008 ) CD151 accelerates breast cancer by regulating alpha 6 integrin function, signaling, and molecular organization . Cancer Res 68 (9) : 3204 – 3213
Yoo SH, Lee K, Chae JY, Moon KC ( 2011 ) CD151 expression can predict cancer progression in clear cell renal cell carcinoma . Histopathology 58 (2) : 191 – 197
Zahir N, Lakins JN, Russell A, Ming W, Chatterjee C, Rozenberg GI, Marinkovich MP, Weaver VM ( 2003 ) Autocrine laminin-5 ligates alpha6beta4 integrin and activates RAC and NFkappaB to mediate anchorage-independent survival of mammary tumors . J Cell Biol 163 (6) : 1397 – 1407
Zhu GH, Huang C, Qiu ZJ, Liu J, Zhang ZH, Zhao N, Feng ZZ, Lv XH ( 2011 ) Expression and prognostic significance of CD151, c-Met, and integrin alpha3/alpha6 in pancreatic ductal adenocarcinoma . Dig Dis Sci 56 (4) : 1090 – 1098
Zijlstra A, Lewis J, Degryse B, Stuhlmann H, Quigley JP ( 2008 ) The inhibition of tumor cell intravasation and subsequent metastasis via regulation of in vivo tumor cell motility by the tetraspanin CD151 . Cancer Cell 13 (3) : 221 – 234
Zoller M ( 2009 ) Tetraspanins: push and pull in suppressing and promoting metastasis . Nat Rev Cancer 9 (1) : 40 – 55
Acknowledgements
We are grateful to Yeon Jin Ko for her technical assistance, which included slide cutting and immunohistochemical analysis, and to Joonghyun Ahn (Biostatistic team of Samsung Biomedical Research Institute) for statistical support. This work was supported by a Global Frontier Project Grant (NRF-M1AXA002-2011-0028404, NRF-M1AXA002-2011-0028391) of the National Research Foundation funded by the Ministry of Education, Science, and Technology of Korea and by a Grant of the Korea Science and Engineering Foundation (KOSEF) funded by the Korea government (MEST) (No. 2009-0064192).
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Kwon, M., Park, S., Choi, J. et al. Clinical significance of CD151 overexpression in subtypes of invasive breast cancer. Br J Cancer 106, 923–930 (2012). https://doi.org/10.1038/bjc.2012.11
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2012.11
Keywords
This article is cited by
-
Cooperative interaction between ERα and the EMT-inducer ZEB1 reprograms breast cancer cells for bone metastasis
Nature Communications (2022)
-
CD151 drives cancer progression depending on integrin α3β1 through EGFR signaling in non-small cell lung cancer
Journal of Experimental & Clinical Cancer Research (2021)
-
Understanding the role of integrins in breast cancer invasion, metastasis, angiogenesis, and drug resistance
Oncogene (2021)
-
Targeting AXL and RAGE to prevent geminin overexpression-induced triple-negative breast cancer metastasis
Scientific Reports (2019)
-
MMP11 and CD2 as novel prognostic factors in hormone receptor-negative, HER2-positive breast cancer
Breast Cancer Research and Treatment (2017)