Key Points
-
Reviews evidence with regard to the characteristics and properties of the EMLA cream.
-
Helps the practitioner understand and identify possible toxicity signs after using EMLA.
-
Relates one of the first reports on the direct toxicity of EMLA on the oral mucosa after topical application.
Abstract
This study reports four cases of mucosa ulceration after a 30-minute application of EMLA (0.3 g) as a topical anaesthetic in dentistry. The subjects returned the next day with a white ulceration and desquamation on the application site. EMLA cream should not be applied to the oral mucosa for 30 minutes.
Similar content being viewed by others
Introduction
EMLA cream, the eutectic mixture of two local anaesthetics – 2.5% lidocaine and 2.5% prilocaine (EMLA®, AstraZeneca) – is a topical anaesthetic indicated to promote superficial anaesthesia prior to dermal procedures such as venipuncture and lumbar puncture.1 Although EMLA is not indicated as a topical anaesthetic agent on the oral mucosa, several authors have reported it as the most effective topical agent in dentistry.2,3,4 Previous studies have demonstrated its efficacy in reducing pain during dental injection,3,4,5 minor gingival surgeries, pocket scaling,6,7,8 restorative procedures2,9 and intraoral soft tissue biopsy.8
The most common adverse effects related to EMLA after cutaneous application are mild local reactions such as oedema, erythema, and transient pallor. However, systemic toxicity, usually associated with high doses of the topical anaesthetic, has been reported, including methaemoglobinaemia and seizures.10,11,12,13,14,15,16 In addition, serious chemical eye injuries have been related in the literature after accidental exposure of the EMLA cream to the eye.17,18
There have been a large number of clinical trials on the use of EMLA in dentistry; however, no serious adverse effects concerning the use of EMLA on oral mucosa have been reported in the literature.
Case report
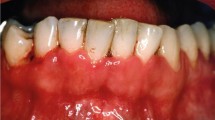
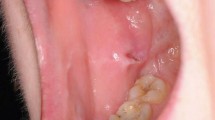
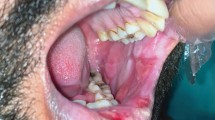
Four healthy females aged between 24 and 44 years (30.5 ± 9.15 years), weighing 56 kg ± 6.32 kg were submitted to topical use of EMLA on the oral mucosa. All of them were in good health and had no history of allergy to the local anaesthetics. They were not taking any medication, except contraceptives (three subjects) as determined by a questionnaire on their health history. The events occurred during a pilot assay that was conducted in order to study the effect of EMLA on oral mucosa. The subjects received 300 mg of the eutectic mixture of 2.5% lidocaine and 2.5% prilocaine cream (EMLA®, AstraZeneca, Cotia, Brazil - EMLA) applied directly on the oral mucosa of the upper right lateral incisor by using a cotton swab for 30 minutes. After application, the oral mucosa was gently wiped with sterile gauze followed by water rinse. The next day after treatment, all subjects complained about pain, a burning sensation and discomfort on the oral mucosa where the topical agent (Fig. 1) was applied. Examination revealed a localised white-ulceration and desquamation of the oral mucosa. The lesion aspect was suggestive of superficial necrosis. All subjects were evaluated daily over one week until the oral mucosa completely recovered. The lesion disappeared three to four days after topical application. At the one-week follow-up visit no visible lesion was observed and no pain or discomfort was reported.
Discussion
Although in some studies2,3,4,19 EMLA cream was indicated as the most effective topical agent for dental use, other studies reported a number of disadvantages, such as a bitter taste (pH = 9.0) and a burning sensation as well as the low viscosity and expensive cost.20 In addition, the manufacturer does not recommend it for oral use because the safe dosing amounts are unknown for mucosa application.21,22 In this case report, all subjects complained about the bitter taste and unpleasant sensation right after EMLA application. Besides the bitter taste, the strong alkalinity has been pointed out as the probable cause of the eye injuries, mainly due to saponification of tissues.18
The use of EMLA is also a matter of concern due to the presence of prilocaine, which can induce methaemoglobinaemia, as reported by the manufacturer. There are an increasing number of methaemoglobinaemia cases reported in the literature caused by EMLA, especially in children undergoing small skin surgeries.10,11,14,15,16 In addition, previous studies demonstrated other side effects after topical application of EMLA on intact skin, such as allergic dermatitis,23 contact urticaria,24 purpura,25 and blanching of tissues.26 EMLA cream also induced an intense inflammatory response damaging the host defences in contaminated wounds.27 Despite those disadvantages and side effects, the use of EMLA has been increasing as a topical anaesthetic in dentistry. To date, there are no reports of serious toxicity after EMLA application on oral soft tissues.
McMillan et al.4 reported no adverse effects in a 24-hour follow up after the application of 0.5 g of EMLA during ten minutes. Using a similar methodology (0.5 g of EMLA during ten minutes of application), Munshi et al.28 evaluated the use of EMLA as an injection free alternative for various clinical procedures, such as extraction of mobile primary teeth, root stumps of primary teeth, incision and drainage of abscesses, restorative and endodontic treatment in children (four to 13 years). These authors found no adverse effects on gingival mucosa after topical application of EMLA. A higher amount of EMLA (8 g during 30 minutes) on the oral mucosa, used to study its pharmacokinetics, showed no local toxicity in any subject,9 although Vickers and Punnia-Moorthy2 reported a slight burning sensation after EMLA application (0.5 g for 30 to 40 minutes) in two out of 25 volunteers.
In the present study, in spite of the use of similar or lower doses than those reported in previous studies2,4,5,8,20,28 during 30 minutes, which is the same application time reported by previous authors,2,9 all subjects showed painful ulceration and desquamation of gingival mucosa. Such factors as different manufacturers, overtime validity and storage conditions were discharged. Incidentally, all subjects were females, but there were no previous reports of toxicity in females in similar studies. There were no apparent reasons to explain the toxicity described in the present study. Further studies are needed to assess possible individual or drug factors that could be responsible for these adverse effects.
References
Parker J F, Vats A, Bauer G . EMLA toxicity after application for allergy skin testing. Pediatrics 2004; 113: 410–411.
Vickers E R, Punnia-Moorthy A . Pulpal anaesthesia from an application of a eutectic topical anaesthetic. Quintessence Int 1993; 24: 547–551.
Roghani S, Duperon D F, Barcohana N . Evaluating the efficacy of commonly used topical anaesthetics. Pediatr Dent 1999; 21: 197–200.
McMillan A S, Walshaw D, Meechan J G . The efficacy of EMLA and 5% lignocaine gel for anaesthesia of human gingival mucosa. Br J Oral Maxillofac Surg 2000; 38: 58–61.
Meechan J G, Thomason J M . A comparison of 2 topical anaesthetics on the discomfort of intraligamentary injections: a double-blind, split-mouth volunteer clinical trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999; 87: 362–365.
Svensson P, Petersen J K, Svensson H . Efficacy of a topical anaesthetic on pain and unpleasantness during scaling of gingival pockets. Anesth Prog 1994; 41: 35–39.
Donaldson D, Meechan J G . A comparison of the effects of EMLA cream and topical 5% lidocaine on discomfort during gingival probing. Anesth Prog 1995; 42: 7–10.
Meechan J G . The use of EMLA for an intraoral soft-tissue biopsy in a needle phobic: a case report. Anesth Prog 2001; 48: 32–34.
Vickers E R, Marzbani N, Gerzina T M, McLean C, Punnia-Moorthy A, Mather L . Pharmacokinetics of EMLA cream 5% application to oral mucosa. Anesth Prog 1997; 44: 32–37.
Rincon E, Baker R L, Iglesias A J, Duarte A M . CNS toxicity after topical application of EMLA cream on a toddler with molluscum contagiosum. Pediatr Emerg Care 2000; 16: 252–254.
Touma S, Jackson J B . Lidocaine and prilocaine toxicity in a patient receiving treatment for mollusca contagiosa. Am Acad Dermatol 2001; 44: 399–400.
Sinisterra S, Miravet E, Alfonso I, Soliz A, Papazian O . Methemoglobinemia in an infant receiving nitric oxide after the use of eutectic mixture of local anaesthetic. J Pediatr 2002; 141: 285–286.
Hahn I H, Hoffman R S, Nelson L S . EMLA-induced methemoglobinaemia and systemic topical anaesthetic toxicity. J Emerg Med 2004; 26: 85–88.
Perez-Caballero Macarron C, Perez Palomino A, Moreno Fernandez L . Probable methemoglobinemia following EMLA administration. Anot Pediatr 2005; 63: 179–180.
Raso S M, Fernandez J B, Beobide E A, Landaluce A F . Methemoglobinemia and CNS toxicity after topical application of EMLA to a 4-year-old girl with molluscum contagiosum. Pediatr Dermatol 2006; 23: 592–593.
Wieringa J W, Ketel A G, van Houten M A . Coma in a child after treatment with the 'magic salve' lidocaine-prilocaine cream. Ned Tijdschr Geneeskd 2006; 150: 1805–1807.
Eaglstein N F . Chemical injury to the eye from EMLA cream during erbium laser resurfacing. Dermatol Surg 1999; 25: 590–591.
McKinlay J R, Hofmeister E, Ross E V, MacAllister W . EMLA cream-induced eye injury. Arch Dermatol 1999; 135: 855–856.
Abu Al-Melh M, Andersson L, Behbehani E . Reduction of pain from needle stick in the oral mucosa by topical anaesthetics: a comparative study between lidocaine/prilocaine and benzocaine. J Clin Dent 2005; 16: 53–56.
Meechan J G, Donaldson D . The intraoral use of EMLA cream in children: a clinical investigation. ASDC J Dent Child 1994; 61: 260–262.
Primosch R E, Rolland-Asensi G . Comparison of topical EMLA 5% oral adhesive to benzocaine 20% on the pain experienced during palatal anaesthetic infiltration in children. Pediatr Dent 2001; 23: 11–14.
Meechan J G . Effective topical anaesthetic agents and techniques. Dent Clin North Am 2002; 46: 759–766.
Suhonen R, Kanerva L . Contact allergy and cross-reactions caused by prilocaine. Am J Contact Dermat 1997; 8: 231–235.
Waton J, Boulanger A, Trechot P H, Schmutz J L, Barbaud A . Contact urticaria from EMLA cream. Contact Dermatitis 2004; 51: 284–287.
Neri I, Savoia F, Guareschi E, Medri M, Patrizi A . Purpura after application of EMLA cream in two children. Pediatr Dermatol 2005; 22: 566–568.
Villada G, Zetlaoui J, Revuz J . Local blanching after epicutaneous application of EMLA cream. A double-blind randomized study among 50 healthy volunteers. Dermatologica 1990; 181: 38–40.
Powell D M, Rodeheaver G T, Foresman P A et al. Damage to tissue defenses by EMLA cream. J Emerg Med 1991; 9: 205–209.
Munshi A K, Hegde A M, Latha R . Use of EMLA: is it an injection free alternative? J Clin Pediatr Dent 2001; 25: 215–219.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Franz-Montan, M., Ranali, J., Ramacciato, J. et al. Ulceration of gingival mucosa after topical application of EMLA: report of four cases. Br Dent J 204, 133–134 (2008). https://doi.org/10.1038/bdj.2008.48
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bdj.2008.48