Abstract
This study (PHANTASTIC) compares first-line plerixafor with granulocyte colony-stimulating factor (G-CSF) in 98 myeloma and lymphoma patients with 151 historic controls mobilised by conventional chemotherapy+G-CSF. Eleven patients developed mild transient symptoms possibly related to plerixafor. No serious adverse events were seen. Seventy (71%) plerixafor-mobilised patients achieved both ⩾4 × 106 CD34+ cells/kg in ⩽2 aphereses and no neutropenia (<1.0 × 109/l). This is significantly >48 (32%) of 151 historical chemotherapy+G-CSF-mobilised control patients achieving this end point (P<0.001). Ninety-six (98%) plerixafor-mobilised patients achieved ⩾2 × 106 CD34+ cells/kg within one harvest round compared with 114 (75%) of controls (P=0.001). Engraftment times and 12-month outcome were comparable in both groups. Prior treatment was summarised by two scoring systems. Controls mobilising either >2.0 or >4.0 × 106 CD34+ cells/kg have significantly lower scores than mobilisation failures (P=0.002), but this relationship was not seen for plerixafor-mobilised patients. Plerixafor is a more effective and less toxic mobilising agent than conventional chemotherapy (especially in heavily pretreated patients), with comparable subsequent outcome, and merits consideration as the first-line standard of care for stem cell mobilisation.
Similar content being viewed by others
Introduction
High-dose chemotherapy with autologous stem cell rescue, colloquially known as autologous stem cell transplantation (SCT), has been in widespread use for over 20 years. At first, bone marrow harvested under general anaesthesia was used as the graft, but it was recognised in the early 1990’s that it is possible to mobilise marrow haematopoietic stem cells (HSCs) into the peripheral blood (PB). PB-derived HSCs may engraft several days faster than those derived from marrow and do not require access to an operating theatre and anaesthetic facilities. Furthermore, it is possible to achieve higher HSCs yields from PB than from marrow, and occasionally these can support two transplants. These factors have resulted in PB HSCs becoming much more widely used than marrow as the graft in almost all HSC centres.
Granulocyte colony-stimulating factor (G-CSF) is widely used as an HSC-mobilising agent. When used alone it may elicit a 10–100-fold increase in circulating HSCs, peaking at 5 days post administration.1 Single agent G-CSF is the standard HSC mobilisation strategy in some centres, especially for myeloma patients in whom HSC mobilisation may be perceived as easier than for lymphoma patients. However, HSC mobilisation using chemotherapy followed by G-CSF is associated with higher yields of HSCs, and is widely used particularly in European HSC centres. The chemotherapy component may be cyclophosphamide or a lymphoma salvage regime such as ESHAP/DHAP. Chemotherapy-containing mobilisation schedules may cause significant neutropenia and nausea over the few days following administration, which may preclude successful harvesting. Furthermore, cyclophosphamide-induced mobilisation is slow, as several leukaphereses may be required until day 12 or even later. For patients with lymphoid malignancy who are harvested following a lymphoma salvage schedule such as ESHAP/DHAP, toxicity is higher and harvesting may not be feasible until the third week following commencement of the chemotherapy. There is, therefore, a need to develop alternative stem cell mobilisation schedules that are independent of the toxicity associated with chemotherapy-containing schedules.
Plerixafor is a bicyclam derivative which is a potent and selective antagonist of the CXCR4 receptor, competing with the latter’s cognate ligand SDF-1α (also known as CXCL12). Plerixafor mobilises human HSC with long-term repopulating ability in immunodeficient mice,2 and acts synergistically with G-CSF for the mobilisation of HSC in both mice and humans. Two multicentre phase III double-blinded placebo-controlled studies of plerixafor have been carried out in patients with lymphoma and myeloma. In the 3101 study, 298 patients with non-Hodgkin lymphoma were randomised to receive either G-CSF 10 μg/kg per day plus plerixafor 240 μg/kg per day or G-CSF+placebo. The primary end point, collection of 5 × 106 CD34+ cells/kg in 4 or fewer days of apheresis, was achieved in 59% of patients on the plerixafor arm compared with 20% in the placebo arm.3 Subsequently, 90% of the plerixafor group and 55% of the placebo group underwent transplantation. No differences were seen in time to platelet or neutrophil engraftment, the durability of the graft out to 12 months follow-up, or the relapse rate or the overall survival. In the 3102 study, the design was the same, but applied to 302 patients with multiple myeloma and with a higher HSC target yield of 6 × 106 CD34+ cells/kg. Similar results were obtained, with 72% of the plerixafor group and 34% of the placebo group achieving the target HSC yield, and after subsequent transplantation, no differences were seen in time to engraftment or in outcome.4 Two further recent studies of first-line plerixafor+G-CSF are consistent with these data.5, 6 Plerixafor+G-CSF is, therefore, a more effective HSC-mobilising schedule than G-CSF alone. The combination allows the collection of greater numbers of stem cells in fewer apheresis sessions than G-CSF alone, and can salvage those patients who fail to mobilise adequate HSC with chemotherapy-based mobilisation or with G-CSF alone.7, 8, 9, 10, 11, 12 However, although there are data on adding plerixafor to chemotherapy+G-CSF mobilisation,13, 14, 15 no studies have investigated how plerixafor+G-CSF as a first-line mobilisation strategy compares with chemotherapy+G-CSF.
Here we report our study of ‘Plerixafor Harvesting And No chemotherapy for Transplantation of Autologous STem cells In Cancer’ (acronym PHANTASTIC), in which patients receive first-line plerixafor with G-CSF. Their harvesting data and subsequent outcome are compared with a historical control group who underwent conventional mobilisation with chemotherapy. We report that plerixafor gives better CD34+ cell yields and is less toxic than conventional chemotherapy, and that there is no difference in subsequent clinical outcome. The data make a strong case for preferring plerixafor+G-CSF to chemotherapy+G-CSF for first-line mobilisation of lymphoma and myeloma patients requiring SCT.
Patients and methods
Study design
PHANTASTIC is registered at http://clinicaltrials.gov (identifier: NCT01186224), and was approved by the Liverpool Central committee of the UK National Research Ethics Service, the UK Medicines and Health Care Regulatory Agency. Between April 2010 and June 2012 (apart from a 3-month interval in 2011 because of temporary unforeseen drug supply problems), trial entry was offered to, and accepted by, all 101 consecutive patients with underlying myeloma or lymphoma aged 18 or over referred to our centre for SCT as their next course of treatment. Plasma cell dyscrasia variants such as light chain deposition disease or amyloidosis, any form of lymphoma or chronic lymphoproliferative disease were all eligible, but patients with plasma cell leukaemia, myeloid malignancy, acute lymphoblastic leukaemia, solid tumours or those undergoing harvesting solely for storage in case of future relapse were not eligible. Patients were ineligible if they had undergone any prior attempt at harvesting for the current transplant.
The treatment protocol comprised G-CSF (filgrastim) for at least 5 days to a maximum of 8 days, and plerixafor commencing daily at 22 h on day 4 for a maximum of four doses. Stem cell harvesting was carried out on day 5 and daily thereafter, until either the target stem cell number (at least 4 × 106 CD34+ cells/kg recipient weight) was collected or four procedures had been carried out. The dose of plerixafor was 240 μg/kg daily if the creatinine clearance (CrCl) was ⩾50 ml/min, or 160 μg/kg daily if the CrCl was 30–49 ml/min. Patients whose CrCl was <30 ml/min were excluded.
The primary study end point was a composite of both an optimal stem cell harvest (at least 4 × 106 CD34+ cells/kg recipient weight in no >2 aphereses) and a neutrophil count that never fell below 1.0 × 109/l in the 3 weeks following initiation of mobilisation. Secondary end points included the usage of plerixafor and the number and timing of apheresis collections, the CD34+ cell yield in each apheresis, serial neutrophil and platelet counts during mobilisation, the time to neutrophil and platelet engraftment after subsequent SCT and the clinical outcome 12 months after SCT. Neutrophil engraftment was defined as the first of at least 2 consecutive days at which the neutrophil count equalled or exceeded 0.5 × 109/l; platelet engraftment as the first of at least 2 consecutive days at which the platelet count equalled or exceeded 50 × 109/l.
The data in these PHANTASTIC patients are compared with those in an immediately preceding unselected consecutive cohort of 151 myeloma and lymphoma patients meeting the same entry criteria as the trial entrants, mobilised with chemotherapy+G-CSF (filgrastim), in whom harvesting was attempted.
Calculation of prior treatment scores
The amount of prior chemoradiotherapy was summarised using an updated version of the ‘original’ scoring system that we reported in 1998,16 initially developed by Drake et al.17 that gives examples of its use for commonly used chemotherapy schedules. Details are as reported previously, though this was updated to encompass more recent chemotherapy agents. In brief, each chemotherapy drug is assigned a toxicity score from 0 (prednisolone and dexamethasone), 1 (vincristine, vinblastine, bleomycin, alpha Interferon, rituximab and brentuximab), 2 (cyclophosphamide, anthracyclines, mitozantrone, cisplatin, etoposide, ifosfamide, cytosine arabinoside, gemcitabine, fludarabine, methotrexate, bortezomib, thalidomide and lenalidomide), 3 (chlorambucil, procarbazine and dacarbazine) to 4 (melphalan, carmustine, mechlorethamine, lomustine and a prior autologous SCT). An additional 2 points are added if mediastinal or treatment dose spinal radiotherapy was given (no points added for palliative radiotherapy for pain control). Intrathecal chemotherapy with either cytosine or methotrexate was scored as 0. The number of courses of each drug received was multiplied by its toxicity factor, and the score for each drug administered was summed to yield an overall treatment score, as previously described.16, 17 We also devised a simplified ‘Liverpool’ scoring system, whereby individual chemotherapy regimes were allocated a score of 1, 2 or 3 according to their myelotoxicity as in Table 1; thus 6 courses of CHOP or R-CHOP would score as 6 × 2=12 points. These updated original and simplified Liverpool scores, together with the total number of treatment courses and regimes, were then each compared for their effect on the resultant yield of CD34+ cells.
Results
Study populations
The PHANTASTIC trial was offered to 101 patients, but 1 of these was found to have relapsed in the interval between being offered the trial and attending for formal consent and screening. In addition, two patients failed screening, one because of renal impairment (CrCl <30 ml/min) and one because the blood film revealed unexpected relapse. Ninety-eight of 100 screened patients, therefore, proceeded to harvest with plerixafor and G-CSF. Of these, 97 received the full plerixafor dose of 240 μg/kg, and 1 received 160 μg/kg because of a CrCl of 47 ml/min.
Table 2 gives demographic details of the 98 harvested PHANTASTIC patients and the 151 historical control patients. The plerixafor and control groups were well matched in terms of age, sex, underlying disease, status at harvest and the extent of prior treatment. Chemotherapy mobilisation regimes in the control patients comprised cyclophosphamide at a dose of 1.5 gm/m2 in 89 patients (all 79 myeloma patients plus 10 lymphoma patients) and various forms of lymphoma salvage chemotherapy in 62 lymphoma patients (details in Table 2).
Plerixafor is more effective than chemotherapy mobilisation
A total of 70 PHANTASTIC patients (71%) achieved the primary end point of at least 4.0 × 106 CD34+cells/kg in either 1 or 2 apheresis collections, and no evidence of neutropenia (defined as <1.0 × 109/l). No patient became neutropenic at any stage during harvesting; the 28 cases that failed the primary end point did so solely because their CD34+ yield was <4.0 × 106 CD34+cells/kg (22 cases) or because they required 3 or 4 aphereses to collect at least this number of cells (6 cases). All but 4 cases achieved an ‘adequate’ harvest of 2.0 × 106 CD34+cells/kg within a total of 4 aphereses. Table 3 sets out the number of cases achieving the primary end point target of 4.0 × 106 CD34+cells/kg, after various numbers of aphereses. Collections of between 3.5 and 4.0 × 106/kg CD34+ cells/kg were achieved in eight patients.
In contrast, only 48 of 151 (32%) control patients passed the primary end point. Forty (26%) patients failed to mobilise at least 4.0 × 106 CD34+ cells/kg, and 67 (44%) became neutropenic (less than 1 × 109/litre); 25 patients (17%) failed both to mobilise at least 4.0 × 106 CD34+ cells/kg and also became neutropenic. Twenty-one cases failed the end point because 3 or 4 aphereses were required to collect the cells. Thirty-seven (25%) control patients failed to achieve an ‘adequate’ harvest of at least 2.0 × 106 CD34+ cells/kg, compared with only 4 (4%) PHANTASTIC patients (P<0.001; 2-sample t-test).
Of the 85 plerixafor-mobilised patients who eventually proceeded to transplant, 79 mobilised adequate (at least 2.0 × 106 CD34+ cells/kg) cells in a single round, and only 3 (4%) required more than one round of harvesting (Table 4; three patients, all with Hodgkins disease, mobilised adequate cells for autografting, but were subsequent scheduled to receive an allograft because of inadequate disease control on PET scanning). In contrast, of 119 conventionally mobilised patients who were ultimately transplanted, 17 (14%) needed additional rounds of harvesting. Sixteen of these required one round (7 underwent marrow harvesting under general anaesthesia; 5 received plerixafor as a second-line agent; 4 received a second round of chemotherapy/G-CSF mobilisation successfully; and 2 a second round of chemotherapy/G-CSF mobilisation which was unsuccessful; both of these went to allograft) and 1 needed 2 additional rounds, by both marrow collection and plerixafor mobilisation.
Plerixafor is less toxic than chemotherapy mobilisation
No serious adverse events were seen in the PHANTASTIC patients during the 3-week observation period following the commencement of the mobilisation schedule. Conversely, 15 (10%) serious adverse events were noted in 14 conventionally mobilised patients, of which 10 were admissions for sepsis associated with neutrophils <1.0 × 109/l (below 0.5 × 109/l in five cases). The remaining serious adverse events were infection without neutropenia (three cases), excessive bleeding from a femoral vein access line site and thrombosis of the superior vena cava. All patients made full recoveries.
Assessment of non-serious adverse events is complicated by the fact that many patients undergoing leukapheresis report symptoms attributable to toxicity of the citrate anticoagulant. Eleven (11%) of PHANTASTIC patients reported mild gastrointestinal symptoms, insomnia and headaches, which may have been plerixafor related; all these resolved within 48 h.
In patients receiving plerixafor-mobilised grafts, the median time to neutrophil and platelet engraftment was respectively 1 and 2 days slower than in recipients of chemotherapy-mobilised grafts (Table 4). This is in line with a previous report,18 and did not confer a clinically important difference, since patients’ time to discharge was typically not rate limited by their engraftment time.
A theoretical concern with plerixafor replacing chemotherapy for mobilisation is that a potential antitumour effect of the chemotherapy is lost; moreover there is in vitro evidence that plerixafor may mobilise malignant haematological cells from a quiescent niche.19, 20, 21 It is therefore plausible that plerixafor mobilisation might result in a higher relapse rate than chemotherapy mobilisation. Table 4 shows that at 12 months after initiation of harvesting, 18 of 86 (21%) plerixafor-mobilised patients had relapsed, with an actuarial 12-month relapse-free survival of 79%. This is comparable to the 29 relapses (20%) in 148 chemotherapy-mobilised controls, whose 12-month relapse-free survival is 80%. There is, therefore, no evidence of a higher relapse rate in plerixafor-mobilised patients than in chemotherapy-mobilised controls.
The effect of prior treatment on CD34± yield
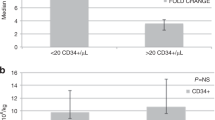
The amount of prior treatment was summarised by four parameters, as defined in the methodology section. These were the updated original chemoradiotherapy score as previously published,16, 17 the simplified Liverpool score (defined in Table 1), the number of courses of treatment and the number of cycles of treatment. Detailed information on prior treatment was available in 97 of the 98 plerixafor-mobilised PHANTASTIC patients and in 142 of the 151 conventionally mobilised patients. PHANTASTIC patients and the control group were well matched for the amount of prior treatment (see Table 2). As shown in Table 5, in the control group, successfully mobilised patients (defined as those achieving at least 2.0 × 106 CD34+ cells/kg) had significantly lower simplified Liverpool treatment scores than those who did not achieve this level (P=0.002, Mann–Whitney) and similar findings were seen for the updated original score and when using a cut off of 4.0 × 106 CD34+ cells/kg. Furthermore, 32 of 36 patients (89%) with updated original scores in the lowest quartile mobilised successfully (at least 2.0 × 106 CD34+ cells/kg), compared with only 25 (69%) with scores in the highest quartile; a similar finding was seen when using the simplified Liverpool score.
In contrast, for PHANTASTIC patients, although those with higher treatment scores tended to mobilise less well, this relationship was not statistically significant, and 24 (100%) and 22 (92%) of 24 patients in the lowest quartiles of updated original scores mobilised at least 2.0 × 106 CD34+ cells/kg.
The total number of treatment courses and cycles were not analysed separately, as these were found to be heavily correlated with both the updated original score and the simplified Liverpool score, as reported previously.16
Discussion
Several previous studies have established that plerixafor+G-CSF is an effective mobilisation strategy and has superior efficacy to G-CSF alone.3, 4, 5, 6 It is also clear that plerixafor+G-CSF is effective as a second-line harvesting strategy where conventional schedules have failed;7, 8, 9, 10, 11, 12 indeed this is covered by the product licence. There is also increasing interest in adding plerixafor to patients currently mobilising poorly with G-CSF±chemotherapy;13, 14, 15 this is variously called pre-emptive or ‘just-in-time’ use, and this strategy has recently been also described for healthy allogeneic donors.22 These observations make clear that plerixafor+G-CSF is an effective mobilising schedule, but how it compares with conventional chemotherapy+G-CSF is not adequately studied. At our institution, 17% of historical myeloma and lymphoma patients fail to collect at least 2.0 × 106 CD34+ cells/kg in up to four aphereses with chemotherapy-based mobilisation schedules; this is similar to data from other centres.23 Their probability of achieving adequate stem cell numbers with either a second harvesting round or with a marrow harvest performed after 4-week rest is low, in line with other reports.23, 24 These patients are, therefore, rarely able to undergo safe autografting, compromising their long-term outcome. Furthermore, at our institution, 30% of patients present in the 3 weeks following initiation of chemotherapy-based mobilisation with a neutrophil count <1.0 × 109/l. These patients require more regular review and follow-up.
Here we initially confirm the observations of several other groups; plerixafor+G-CSF is an effective first-line strategy, whereby 78% mobilise at least 4.0 × 106 CD34+ cells/kg (71% in 1 or 2 aphereses), and 96% mobilise a minimum cell dose of at least 2.0 × 106 CD34+ cells/kg. These results are superior to those mobilised by conventional chemotherapy-based schedules. They are also achieved without any significant toxicity, in sharp contrast to the 7% incidence of neutropenic sepsis requiring hospital admission that is seen after chemotherapy-based mobilisation. As in several previous studies, we confirm that plerixafor-mobilised cells can safely support an autograft. The present data are in line with the recent report of the PREDICT study.5 This investigated the safety and efficacy of first-line plerixafor+G-SCF in 118 patients across Europe, most of those had underlying myeloma. Plerixafor-related adverse events were transient and mild, as here, and the vast majority of patients mobilised an adequate number of cells (>2.0 × 106 CD34+cells/kg), with most myeloma patients yielding enough cells for two transplants.
We then assessed the efficacy of plerixafor in relation to prior treatment, which has not been previously investigated. Using two different scoring systems to summarise previous treatment (an updated version of our previous scoring system, and also a much simplified novel ‘Liverpool’ score), we show that although heavy previous treatment can prejudice successful harvesting with conventional chemotherapy, such patients may still mobilise well with plerixafor. Attempts have been made to identify poor mobilisers prospectively, to justify their receipt of first-line plerixafor rather than conventional chemotherapy mobilisation. Heavy prior therapy may prejudice successful harvesting, and treatment with purine analogues and three or more previous chemotherapy lines are predictive factors for poor mobilisation;25 these all lead to a high score in both our scoring systems. In such cases, the administration of immediate or pre-emptive plerixafor could be useful to avoid the need for a second round of mobilisation.
The present study is subject to the limitations inherent in using a historical control group, and we acknowledge that a randomised prospective design would strengthen our conclusions. With this in mind, the data, nevertheless, support the view that heavily pretreated cases should receive plerixafor, ideally as a first-line agent to avoid the excess toxicity associated with chemotherapy in ‘just-in-time’ strategies. However, it is not easy to define a score value below which patients may still mobilise well with chemotherapy-based schedules, and the excess toxicity of the latter supports the view that all patients should receive first-line plerixafor+G-CSF, irrespective of their prior treatment. However, plerixafor is an expensive drug and it is important to establish whether it is cost effective. For example, HSC harvests from plerixafor mobilisation may have a lower CD34+ to mononuclear cell ratio, resulting in greater storage costs.26 On the other hand, fewer patients mobilise successfully with chemotherapy-based schedules, and these require reharvesting which consumes additional resource; a proportion may still not mobilise enough cells and cannot, therefore, undergo autografting with a higher risk of relapse and its attendant retreatment costs.
In a retrospective study of the Expanded Access Programme of first-line mobilisation with plerixafor+G-CSF, Shaughnessy et al.27 compared outcomes in 33 US patients to 33 matched historic controls mobilised with cyclophosphamide 3–5 g/m2 and G-CSF at two centres. The median total cost of mobilisation was not significantly different between the plerixafor+G-CSF and control groups ($14 224 versus $18 824). In a study evaluating the costs of a ‘just-in-time’ plerixafor strategy, its use in selected high-risk patients and poor mobilisers did not increase the total charges associated with stem cell collection when compared with poor mobilisers treated with G-CSF alone.28 A cost-benefit analysis of pre-emptive use of plerixafor in patients mobilised by G-CSF alone estimated that this strategy was associated with savings of $19 300 per patient, suggesting that addition of plerixafor to G-CSF significantly reduces the frequency of mobilisation failures and is also cost effective,29 though a separate study of two pre-emptive strategies confirmed efficacy but not lower overall costs.30 In contrast, two recent health economic analyses from the same group suggest that cyclophosphamide, at both 3–4 gm/m2 and 1.5 gm/m2, plus G-CSF may be more cost effective than plerixafo+G-CSF in myeloma patients.31, 32 However, it is unclear whether these analyses took into account the extra costs of repeat harvesting in the additional harvest failures in the chemotherapy-mobilised group, and several of these cost effectiveness analyses used median US costs rather than individual actual patient data.
In summary, we report that plerixafor mobilisation has superior efficacy and lower toxicity compared with conventional chemotherapy, and is effective irrespective of prior treatment intensity. The data support the emerging case for first-line plerixafor as the standard of care for HSC mobilisation, but it is not yet clear whether this is cost effective. Further, health economic analyses of the full costs of both plerixafor- and chemotherapy-based schedules are required, using individual actual patient data in a variety of health care settings.
References
Dreger P, Haferlach T, Eckstein V, Jacobs S, Suttorp M, Löffler H et al. G-CSF-mobilized peripheral blood progenitor cells for allogeneic transplantation: safety, kinetics of mobilization, and composition of the graft. Br J Haematol 1994; 87: 609–613.
Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med 2005; 201: 1307–1318.
DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E et al. 3101 Investigators. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol 2009; 27: 4767–4773.
DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL et al. 3102 Investigators. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood 2009; 113: 5720–5726.
Russell N, Douglas K, Ho AD, Mohty M, Carlson K, Ossenkoppele GJ et al. Plerixafor and granulocyte colony-stimulating factor for first-line steady-state autologous peripheral blood stem cell mobilization in lymphoma and multiple myeloma: results of the prospective PREDICT trial. Haematologica 2013; 98: 172–178.
Shaughnessy P, Uberti J, Devine S, Maziarz RT, Vose J, Micallef I et al. Plerixafor and G-CSF for autologous stem cell mobilization in patients with NHL, Hodgkin's lymphoma and multiple myeloma: results from the expanded access program. Bone Marrow Transplantation 2013; 48: 777–781.
Calandra G, McCarty J, McGuirk J, Tricot G, Crocker SA, Badel K et al. AMD3100 plus G-CSF can successfully mobilize CD34+ cells from non-Hodgkin's lymphoma, Hodgkin's disease and multiple myeloma patients previously failing mobilization with chemotherapy and/or cytokine treatment: compassionate use data. Bone Marrow Transplantation 2008; 41: 331–338.
Uy GL, Rettig MP, Cashen AF . Plerixafor, a CXCR4 antagonist for the mobilization of hematopoietic stem cells. Expert Opin Biol Ther 2008; 8: 1797–1804.
Fowler CJ, Dunn A, Hayes-Lattin B, Hansen K, Hansen L, Lanier K et al. Rescue from failed growth factor and/or chemotherapy HSC mobilization with G-CSF and plerixafor (AMD3100): an institutional experience. Bone Marrow Transplantation 2009; 43: 909–917.
Duarte RF, Shaw BE, Marín P, Kottaridis P, Ortiz M, Morante C et al. Plerixafor plus granulocyte CSF can mobilize hematopoietic stem cells from multiple myeloma and lymphoma patients failing previous mobilization attempts: EU compassionate use data. Bone Marrow Transplantation 2011; 46: 52–58.
Arcaini L, Laszlo D, Rizzi S, Balzarotti M, Antoniazzi F, Zilioli VR et al. Plerixafor and G-CSF for PBSC mobilization in patients with lymphoma who failed previous attempts with G-CSF and chemotherapy: a REL (Rete EmatologicaLombarda) experience. Leuk Res 2011; 35: 712–714.
Abhyankar S, DeJarnette S, Aljitawi O, Ganguly S, Merkel D, McGuirk J . A risk-based approach to optimize autologous hematopoietic stem cell (HSC) collection with the use of plerixafor. Bone Marrow Transplantation 2012; 47: 483–487.
Dugan MJ, Maziarz RT, Bensinger WI, Nademanee A, Liesveld J, Badel K et al. Safety and preliminary efficacy of plerixafor (Mozobil) in combination with chemotherapy and G-CSF: an open-label, multicenter, exploratory trial in patients with multiple myeloma and non-Hodgkin's lymphoma undergoing stem cell mobilization. Bone Marrow Transplantation 2010; 45: 39–47.
Hübel K, Fresen MM, Salwender H, Basara N, Beier R, Theurich S et al. Plerixafor with and without chemotherapy in poor mobilizers: results from the German compassionate use program. Bone Marrow Transplantation 2011; 46: 1045–1052.
D'Addio A, Curti A, Worel N, Douglas K, Motta MR, Rizzi S et al. The addition of plerixafor is safe and allows adequate PBSC collection in multiple myeloma and lymphoma patients poor mobilizers after chemotherapy and G-CSF. Bone Marrow Transplantation 2011; 46: 356–363.
Clark RE, Brammer CG . Previous treatment predicts the efficiency of blood progenitor cell mobilisation: validation of a chemotherapy scoring system. Bone Marrow Transplantation 1998; 22: 859–863.
Drake M, Ranaghan L, Morris TC, Nolan L, Desai ZR, Irvine AE et al. Analysis of the effect of prior therapy on progenitor cell yield: use of a chemotherapy scoring system. Br J Haematol 1997; 98: 745–749.
Alexander ET, Towery JA, Miller AN, Kramer C, Hogan KR, Squires JE et al. Beyond CD34+ cell dose: impact of method of peripheral blood hematopoietic stem cell mobilization (granulocyte-colony-stimulating factor [G-CSF], G-CSF plus plerixafor, or cyclophosphamide G-CSF/granulocyte-macrophage [GM]-CSF) on number of colony-forming unit-GM, engraftment, and Day +100 hematopoietic graft function. Transfusion 2011; 51: 1995–2000.
Tavor S, Eisenbach M, Jacob-Hirsch J, Golan T, Petit I, Benzion K et al. The CXCR4 antagonist AMD3100 impairs survival of human AML cells and induces their differentiation. Leukemia 2008; 22: 2151–2158.
Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood 2009; 113: 6206–6214.
Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood 2009; 113: 4341–4351.
Hauge AW1, Haastrup EK, Sengeløv H, Minulescu L, Dickmeiss E, Fischer-Nielsen A . Addition of plerixafor for CD34+ cell mobilization in six healthy stem cell donors ensured satisfactory grafts for transplantation. Transfusion 2014; 54: 1055–1058.
Pusic I, Jiang SY, Landua S, Uy GL, Rettig MP, Cashen AF et al. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood and Marrow Transplantation 2008; 14: 1045–1056.
Watts MJ, Ings SJ, Flynn M, Dodds D, Goldstone AH, Linch DC . Remobilization of patients who fail to achieve minimal progenitor thresholds at the first attempt is clinically worthwhile. Br J Haematol 2000; 111: 287–291.
Sancho JM, Morgades M, Grifols JR, Juncà J, Guardia R, Vives S et al. Predictive factors for poor peripheral blood stem cell mobilization and peak CD34(+) cell count to guide pre-emptive or immediate rescue mobilization. Cytotherapy 2012; 14: 823–829.
Tanhehco YC, Adamski J, Sell M, Cunningham K, Eisenmann C, Magee D et al. Plerixafor mobilization leads to a lower ratio of CD34+ cells to total nucleated cells which results in greater storage costs. J Clin Apheresis 2010; 25: 202–208.
Shaughnessy P, Islas-Ohlmayer M, Murphy J, Hougham M, MacPherson J, Winkler K et al. Cost and clinical analysis of autologous hematopoietic stem cell mobilization with G-CSF and plerixafor compared to G-CSF and cyclophosphamide. Biol Blood Marrow Transplantation 2011; 17: 729–736.
Li J, Hamilton E, Vaughn L, Graiser M, Renfroe H, Lechowicz MJ et al. Effectiveness and cost analysis of "just-in-time" salvage plerixafor administration in autologous transplant patients with poor stem cell mobilization kinetics. Transfusion 2011; 10: 2175–2182.
Vishnu P, Roy V, Paulsen A, Zubair AC . Efficacy and cost-benefit analysis of risk-adaptive use of plerixafor for autologous hematopoietic progenitor cell mobilization. Transfusion 2012; 52: 55–62.
Micallef IN, Sinha S, Gastineau DA, Wolf R, Inwards DJ, Gertz MA et al. Cost-effectiveness analysis of a risk-adapted algorithm of plerixafor use for autologous peripheral blood stem cell mobilization. Biol Blood Marrow Transplantation 2013; 19: 87–93.
Awan F, Kochuparambil ST, Falconer DE, Cumpston A, Leadmon S, Watkins K et al. Comparable efficacy and lower cost of PBSC mobilization with intermediate-dose cyclophosphamide and G-CSF compared with plerixafor and G-CSF in patients with multiple myeloma treated with novel therapies. Bone Marrow Transplantation 2013; 48: 1279–1284.
Chaudhary L, Awan F, Cumpston A, Leadmon S, Watkins K, Tse W et al. Peripheral blood stem cell mobilization in multiple myeloma patients treat in the novel therapy-era with plerixafor and G-CSF has superior efficacy but significantly higher costs compared to mobilization with low-dose cyclophosphamide and G-CSF. J Clin Apheresis 2013; 28: 359–367.
Acknowledgements
The study was conceived by REC, who was in overall charge of the study including regulatory issues, and co-wrote the manuscript. UV recruited patients, derived data and contributed to the manuscript. RS was in charge of clinical care and co-wrote the manuscript. JB was responsible for its day to day running. BB managed the overall data, and JOC derived follow-up and other patient data. SF and RS recruited patients. NMc and TC were responsible for stem cell harvesting. UV, BB and REC analysed the data.
Author Contributions
The study was sponsored jointly by the University of Liverpool and the Royal Liverpool University Hospital. Support for infrastructure and trial drug was provided by Sanofi (formerly Genzyme), which we gratefully acknowledge.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Clark, R., Bell, J., Clark, J. et al. Plerixafor is superior to conventional chemotherapy for first-line stem cell mobilisation, and is effective even in heavily pretreated patients. Blood Cancer Journal 4, e255 (2014). https://doi.org/10.1038/bcj.2014.79
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bcj.2014.79
This article is cited by
-
Chemotherapy-based versus chemotherapy-free stem cell mobilization (± plerixafor) in multiple myeloma patients: an Italian cost-effectiveness analysis
Bone Marrow Transplantation (2021)
-
A single center’s experience using four different front line mobilization strategies in lymphoma patients planned to undergo autologous hematopoietic cell transplantation
Bone Marrow Transplantation (2017)
-
A randomized phase II study of stem cell mobilization with cyclophosphamide+G-CSF or G-CSF alone after lenalidomide-based induction in multiple myeloma
Bone Marrow Transplantation (2016)